
The IUPAC name of Glycerin is:
A. Glycerol
B. 1,2-ethanediol
C. Propane-1,2,3-triol
D. 1,2,3-trihydroxypropane
Answer
609k+ views
Hint: In IUPAC nomenclature, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the types of the functional groups present on (or within) the parent chain. Other groups which are attached to the parent chain are called substituents.
Complete step by step answer:
Glycerin- Glycerin is also known as Glycerol. Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic.
Structure of Glycerin -
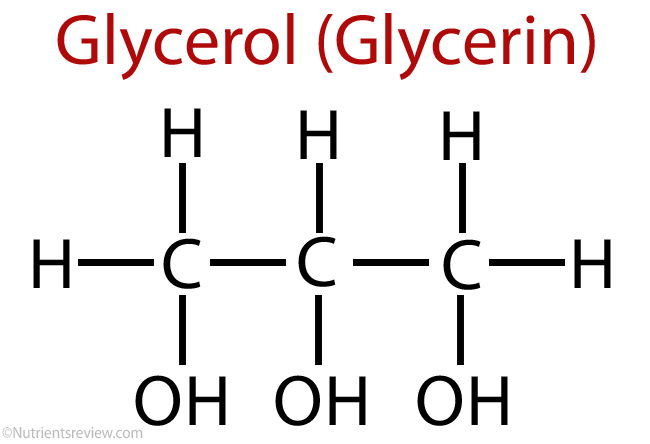
IUPAC Nomenclature of compound -
As we know that the base part of the name reflects the number of carbons in what we have assigned to be the parent chain. There are a total three carbons present in the parent chain, so it is a propane.
All three carbons are attached to the same functional group, that is alcohol. Since it is the only functional group present, it will be used as a prefix in the name.
It is a symmetric compound, so we can name it from either side, left or right. We are naming it from left to right.
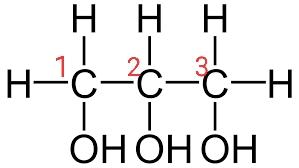
For three carbons, we will use propane
For three alcohol group substituents, we will use -triol as a suffix.
For position of alcohol functional group, we will use 1,2,3
Now we are all set to write the IUPAC name of Glycerin, that is Propane-1,2,3-triol
Hence the correct option is C.
Note: In IUPAC nomenclature always separate the number from another number with a comma ( , ) and a number from a word with a dash ( - )
Also not confuse yourself with option D, 1,2,3-trihydroxypropane. Here alcohol group is used as a prefix, which is the wrong way to do because IUPAC suggests to use the main functional group as a suffix in the name.
Complete step by step answer:
Glycerin- Glycerin is also known as Glycerol. Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic.
Structure of Glycerin -

IUPAC Nomenclature of compound -
As we know that the base part of the name reflects the number of carbons in what we have assigned to be the parent chain. There are a total three carbons present in the parent chain, so it is a propane.
All three carbons are attached to the same functional group, that is alcohol. Since it is the only functional group present, it will be used as a prefix in the name.
It is a symmetric compound, so we can name it from either side, left or right. We are naming it from left to right.

For three carbons, we will use propane
For three alcohol group substituents, we will use -triol as a suffix.
For position of alcohol functional group, we will use 1,2,3
Now we are all set to write the IUPAC name of Glycerin, that is Propane-1,2,3-triol
Hence the correct option is C.
Note: In IUPAC nomenclature always separate the number from another number with a comma ( , ) and a number from a word with a dash ( - )
Also not confuse yourself with option D, 1,2,3-trihydroxypropane. Here alcohol group is used as a prefix, which is the wrong way to do because IUPAC suggests to use the main functional group as a suffix in the name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




