
The IUPAC name of \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\] is:
A.methylamine
B.amino ethane
C.methanamine
D.ethylamine
Answer
585.9k+ views
Hint:Identify the longest chain of the carbon as the root. The main functional group is to be written as a suffix and the substitute is written as a prefix. The sequence of the naming should be in the way:
Prefix + root + suffix
Complete step by step answer:
The longest chain contains one carbon atom and one N atom. So it is the root. Since the compound contains a \[{\text{N}}{{\text{H}}_{\text{2}}}\] molecule and a \[{\text{C}}{{\text{H}}_3}\]molecule, hence the name would contain amine and methyl.
We now assign numbers to the carbon and the nitrogen atom in the following way,
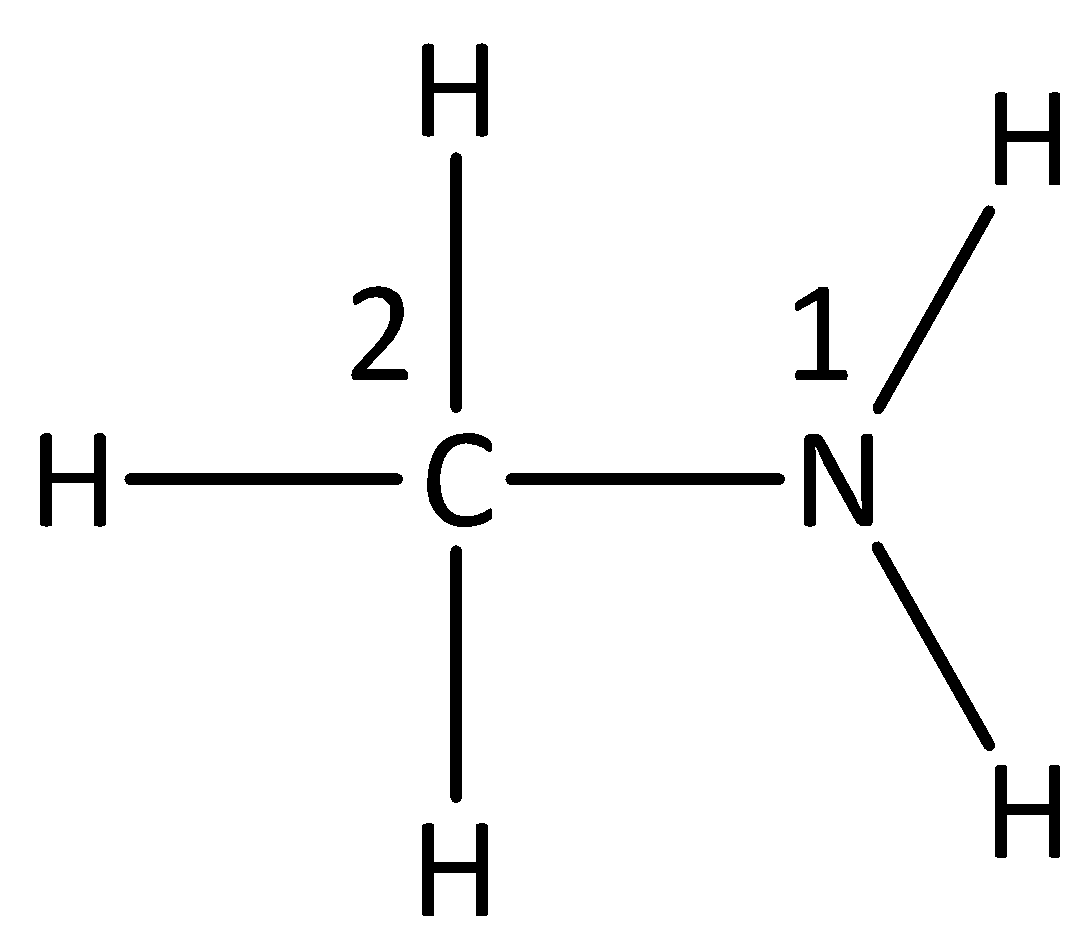
In the compound, we see that the 1 position contains the \[{\text{N}}{{\text{H}}_{\text{2}}}\] group and the 2 position contains the \[{\text{C}}{{\text{H}}_3}\] group.
From this we can conclude that the name of the compound will be methylamine.
Therefore, out of the given options, A is the correct answer.
Additional information:
Methylamine is a simple primary amine. This compound is an organic colorless compound. Commercially methylamine can be prepared by the reaction of methanol with that of ammonia.
\[{\text{C}}{{\text{H}}_3}{\text{OH}} + {\text{N}}{{\text{H}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Note:
The rule set by the International union of pure and applied chemistry (IUPAC) must always be followed. For naming, first identify the main chain and then number the carbon atoms in the chain. Secondly, identify the substituents and then name the compound using suffix and prefix. The counting of the number of carbon atoms is always done from the side in which the main functional group is assigned with a lesser number.
Prefix + root + suffix
Complete step by step answer:
The longest chain contains one carbon atom and one N atom. So it is the root. Since the compound contains a \[{\text{N}}{{\text{H}}_{\text{2}}}\] molecule and a \[{\text{C}}{{\text{H}}_3}\]molecule, hence the name would contain amine and methyl.
We now assign numbers to the carbon and the nitrogen atom in the following way,

In the compound, we see that the 1 position contains the \[{\text{N}}{{\text{H}}_{\text{2}}}\] group and the 2 position contains the \[{\text{C}}{{\text{H}}_3}\] group.
From this we can conclude that the name of the compound will be methylamine.
Therefore, out of the given options, A is the correct answer.
Additional information:
Methylamine is a simple primary amine. This compound is an organic colorless compound. Commercially methylamine can be prepared by the reaction of methanol with that of ammonia.
\[{\text{C}}{{\text{H}}_3}{\text{OH}} + {\text{N}}{{\text{H}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
Note:
The rule set by the International union of pure and applied chemistry (IUPAC) must always be followed. For naming, first identify the main chain and then number the carbon atoms in the chain. Secondly, identify the substituents and then name the compound using suffix and prefix. The counting of the number of carbon atoms is always done from the side in which the main functional group is assigned with a lesser number.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




