
The IUPAC name of $C{H_3}COCH{\left( {C{H_3}} \right)_2}$ is:
A: $4 - $methyl isopropyl ketone
B: $3 - $methyl$ - 2 - $butanone
C: Isopropyl Methyl ketone
D: $2 - $methyl$ - 3 - $butanone
Answer
586.2k+ views
Hint: There are some rules on the basis of which names are given to the organic compounds. These names are decided on the basis of IUPAC system. In this system, the prefix of the compound name indicates the number of carbon atoms and the suffix of the compound indicates the number of bonds between the carbon atoms.
Complete step by step answer:
In this question we have to find the IUPAC name of $C{H_3}COCH{\left( {C{H_3}} \right)_2}$. Structure of this compound is:
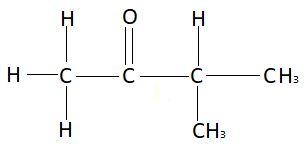
In this compound there are a total four carbon atoms in the main chain. Due to these four carbon atoms in the main chain the prefix used will be but. Between all the carbon atoms there is a single bond, so the suffix used will be ane to represent these single bonds. A functional group and an extra methyl group is also present in this compound. According to the rules, numbering will start from that side from where the functional group gets the lowest number. Functional group will get the lowest number from the left side. So, the numbering will start from the left side. From this side the function group is present at second carbon atom and methyl group is present at third carbon atom. Functional group is ketone in this compound. To represent ketone as a functional group, e of ane will be replaced with one. So, the IUPAC name of this compound will be $3 - $methyl$ - 2 - $butanone as the functional group is in the second carbon atom and the functional group is in the third carbon atom.
So, the correct answer is Option B .
Note:
There are different suffixes used to represent bonds between carbon atoms. Suffix used to represent single bond is ane, to represent double bond suffix used is ene and yne is used to represent triple bond between two carbon atoms.
Complete step by step answer:
In this question we have to find the IUPAC name of $C{H_3}COCH{\left( {C{H_3}} \right)_2}$. Structure of this compound is:

In this compound there are a total four carbon atoms in the main chain. Due to these four carbon atoms in the main chain the prefix used will be but. Between all the carbon atoms there is a single bond, so the suffix used will be ane to represent these single bonds. A functional group and an extra methyl group is also present in this compound. According to the rules, numbering will start from that side from where the functional group gets the lowest number. Functional group will get the lowest number from the left side. So, the numbering will start from the left side. From this side the function group is present at second carbon atom and methyl group is present at third carbon atom. Functional group is ketone in this compound. To represent ketone as a functional group, e of ane will be replaced with one. So, the IUPAC name of this compound will be $3 - $methyl$ - 2 - $butanone as the functional group is in the second carbon atom and the functional group is in the third carbon atom.
So, the correct answer is Option B .
Note:
There are different suffixes used to represent bonds between carbon atoms. Suffix used to represent single bond is ane, to represent double bond suffix used is ene and yne is used to represent triple bond between two carbon atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




