
The IUPAC name of $C{H_3}C{H_2}COCl$ is:
A: propanoyl chloride
B: ethanoyl chloride
C: acetyl chloride
D: chloroethane
Answer
586.2k+ views
Hint: There are some rules on the basis of which names are given to the organic compounds. These names are decided on the basis of IUPAC system. In this system, the prefix of the compound name indicates the number of carbon atoms and the suffix of the compound indicates the number of bonds between the carbon atoms.
Complete step by step answer:
General formula of acid chlorides is $RCOCl$ where, $R$ is alkyl group. Acid chlorides can’t make hydrogen bonding. Due to lack of hydrogen bonding the melting and boiling point of such chlorides is less. In such chlorides the suffix used is oyl chloride. In this question we have to find the IUPAC name of $C{H_3}C{H_2}COCl$. Structure of this compound is as follows:
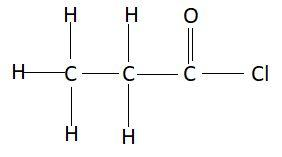
In this compound, there are a total three carbon atoms in the main chain. So, the prefix for this compound will be prop. Since there is a single bond between the carbon atoms suffix for this compound will be ane. Functional group is also present in this compound. To represent a functional group, the suffix that is an will be replaced with the suffix which is used to represent the functional group that is present in this compound. The functional group present in this compound is acid chloride that is $RCOCl$ and oyl chloride is used as a suffix to represent this functional group. Therefore the suffix that will be used in this compound is anoyl chloride. Combining the prefix and suffix, the name of this compound is propanoyl chloride.
So, the correct answer is Option A .
Note:
There are different suffixes used to represent bonds between carbon atoms. Suffix used to represent single bond is ane, to represent double bond suffix used is ene and yne is used to represent triple bond between two carbon atoms.
Complete step by step answer:
General formula of acid chlorides is $RCOCl$ where, $R$ is alkyl group. Acid chlorides can’t make hydrogen bonding. Due to lack of hydrogen bonding the melting and boiling point of such chlorides is less. In such chlorides the suffix used is oyl chloride. In this question we have to find the IUPAC name of $C{H_3}C{H_2}COCl$. Structure of this compound is as follows:

In this compound, there are a total three carbon atoms in the main chain. So, the prefix for this compound will be prop. Since there is a single bond between the carbon atoms suffix for this compound will be ane. Functional group is also present in this compound. To represent a functional group, the suffix that is an will be replaced with the suffix which is used to represent the functional group that is present in this compound. The functional group present in this compound is acid chloride that is $RCOCl$ and oyl chloride is used as a suffix to represent this functional group. Therefore the suffix that will be used in this compound is anoyl chloride. Combining the prefix and suffix, the name of this compound is propanoyl chloride.
So, the correct answer is Option A .
Note:
There are different suffixes used to represent bonds between carbon atoms. Suffix used to represent single bond is ane, to represent double bond suffix used is ene and yne is used to represent triple bond between two carbon atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




