
The IUPAC name of $C{H_3} - C{H_2} - CH\left( {C{H_3}} \right) - C\left( {{C_4}{H_9}} \right)\left( {C{H_3}} \right) - C{H_3}$ is:
A. ${\rm{3,4,4 - trimethyloctane}}$
B. ${\rm{3,4,4 - trimethylheptane}}$
C. ${\rm{2 - ethyl - 3,3 - dimethylheptane}}$
D. ${\rm{2 - butyl - 2 - methyl - 3 - ethylbutane}}$
Answer
578.7k+ views
Hint: We can deduce the IUPAC name of an organic compound by using a given set of rules.
Complete step by step answer:
We can write the IUPAC name of the organic compounds belonging to alkane family, by following some given rules which are listed below:
Firstly, we need to write down the structure of the compound by using the given formula. Here, we can see in the first glance that a five-carbon chain is present in which one \[C{H_3} - \]group is attached to the third carbon atom and one \[C{H_3} - \] group is attached to the fourth carbon atom. In addition to these, one \[{C_4}{H_9} - \] group is attached to the fourth carbon as well. We can draw the structure based on this information:
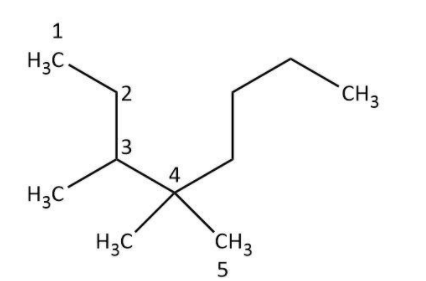
Next, we will identify the longest carbon chain. In the given compound the longest carbon chain has eight carbon atoms not five:
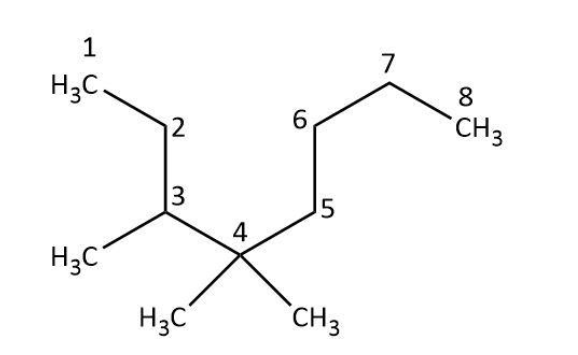
Now, we will identify the root names for the carbon chain and the substituents based on the number of carbon atoms. In \[C{H_3} - \] group, there is only one carbon atom. So, we can derive its name from meth- to be a methyl group. The longest carbon chain has eight carbons for which the root name is oct- and the derived name would be octane.
In the next step, we will assign the positions to the substituents based on the number of carbon atom to which they are attached. In the given compound, we can see that one methyl group is present at the third carbon and two methyl groups are attached to the fourth carbon on the basis of above numbering.
Finally, we can use the above collected information to write the IUPAC name of the compound to be ${\rm{3,4,4 - trimethyloctane}}$.
Thus, the correct option is A.
Note:
We have to be careful while numbering the branched chains as there are multiple possibilities.
Complete step by step answer:
We can write the IUPAC name of the organic compounds belonging to alkane family, by following some given rules which are listed below:
Firstly, we need to write down the structure of the compound by using the given formula. Here, we can see in the first glance that a five-carbon chain is present in which one \[C{H_3} - \]group is attached to the third carbon atom and one \[C{H_3} - \] group is attached to the fourth carbon atom. In addition to these, one \[{C_4}{H_9} - \] group is attached to the fourth carbon as well. We can draw the structure based on this information:

Next, we will identify the longest carbon chain. In the given compound the longest carbon chain has eight carbon atoms not five:

Now, we will identify the root names for the carbon chain and the substituents based on the number of carbon atoms. In \[C{H_3} - \] group, there is only one carbon atom. So, we can derive its name from meth- to be a methyl group. The longest carbon chain has eight carbons for which the root name is oct- and the derived name would be octane.
In the next step, we will assign the positions to the substituents based on the number of carbon atom to which they are attached. In the given compound, we can see that one methyl group is present at the third carbon and two methyl groups are attached to the fourth carbon on the basis of above numbering.
Finally, we can use the above collected information to write the IUPAC name of the compound to be ${\rm{3,4,4 - trimethyloctane}}$.
Thus, the correct option is A.
Note:
We have to be careful while numbering the branched chains as there are multiple possibilities.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




