
The IUPAC name of ${{\text{C}}_{6}}{{\text{H}}_{4}}\text{COCl}$is:
A. Benzoyl chloride
B. Benzene chloro ketone
C. Benzene carbonyl chloride
D. Chlorophenyl ketone
Answer
576.3k+ views
Hint: Benzene is a six-carbon containing compound. The naming of carbon is done according to the amount of it present in the molecule. Ketone is a -CO functional group whereas while doing the naming of halogens, they are commonly named as chloride, fluoride, bromide, etc.
Complete step by step solution:
- In the given question we have to explain the IUPAC or International Union of Applied and Pure Chemistry of the given compound.
- So, the structure of the compound is:
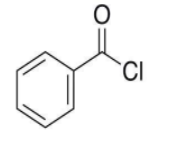
- Here, we can see that a chlorine group is attached at the benzoyl group i.e. ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO-}$.
- We know that the IUPAC name of chlorine is chloride.
- So, the IUPAC name of the above compound is Benzoyl chloride.
- In benzene chloro ketone, the chlorine is attached to the other carbon other than carbonyl carbon.
- So, this option (b) is not correct because the benzoyl group is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO-}$.
- In option (c), the carbonyl group is mentioned whose formula is C=O, the particular group is present in the compound, but it cannot be considered as a prior group,
- So, option (c) is also incorrect.
- A phenyl group is similar to the benzene except it has one hydrogen atom less than the benzene.
- In the phenyl group, one hydrogen is absent and other elements can attach to its place.
- So, option (d) is also incorrect.
Therefore, option (a) is the correct answer.
Note: One may get confused with the benzene, benzyl and benzyl group. There is a difference between these three structures. The formula of benzene, benzyl and benzoyl is ${{\text{C}}_{6}}{{\text{H}}_{5}},\ {{\text{C}}_{6}}{{\text{H}}_{5}}\text{CH}_{2}^{-},{{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO}$ respectively.
Complete step by step solution:
- In the given question we have to explain the IUPAC or International Union of Applied and Pure Chemistry of the given compound.
- So, the structure of the compound is:

- Here, we can see that a chlorine group is attached at the benzoyl group i.e. ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO-}$.
- We know that the IUPAC name of chlorine is chloride.
- So, the IUPAC name of the above compound is Benzoyl chloride.
- In benzene chloro ketone, the chlorine is attached to the other carbon other than carbonyl carbon.
- So, this option (b) is not correct because the benzoyl group is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO-}$.
- In option (c), the carbonyl group is mentioned whose formula is C=O, the particular group is present in the compound, but it cannot be considered as a prior group,
- So, option (c) is also incorrect.
- A phenyl group is similar to the benzene except it has one hydrogen atom less than the benzene.
- In the phenyl group, one hydrogen is absent and other elements can attach to its place.
- So, option (d) is also incorrect.
Therefore, option (a) is the correct answer.
Note: One may get confused with the benzene, benzyl and benzyl group. There is a difference between these three structures. The formula of benzene, benzyl and benzoyl is ${{\text{C}}_{6}}{{\text{H}}_{5}},\ {{\text{C}}_{6}}{{\text{H}}_{5}}\text{CH}_{2}^{-},{{\text{C}}_{6}}{{\text{H}}_{5}}\text{CO}$ respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




