
The IUPAC name of below is:${{(C{{H}_{3}})}_{2}}CH-CH=CH-CH=CH-CH({{C}_{2}}{{H}_{5}})-C{{H}_{3}}$
The IUPAC name of this is:
(A) 2,7-dimethyl-3,5-nonadiene
(B) 2,7-dimethyl-2-methylbutadiene
(C) 2-methyl-7-ethyl-3,5-octadiene
(D) 1,1-dimenthyl-6-ethyl-2,4-heptadiene
Answer
531.9k+ views
Hint: International union of pure and applied chemistry governs the nomenclature of organic chemical compounds. The name of a compound should be able to be used to depict and determine the structural formula of a chemical compound.
Complete answer:
Let us first redraw the compound for better clarity.
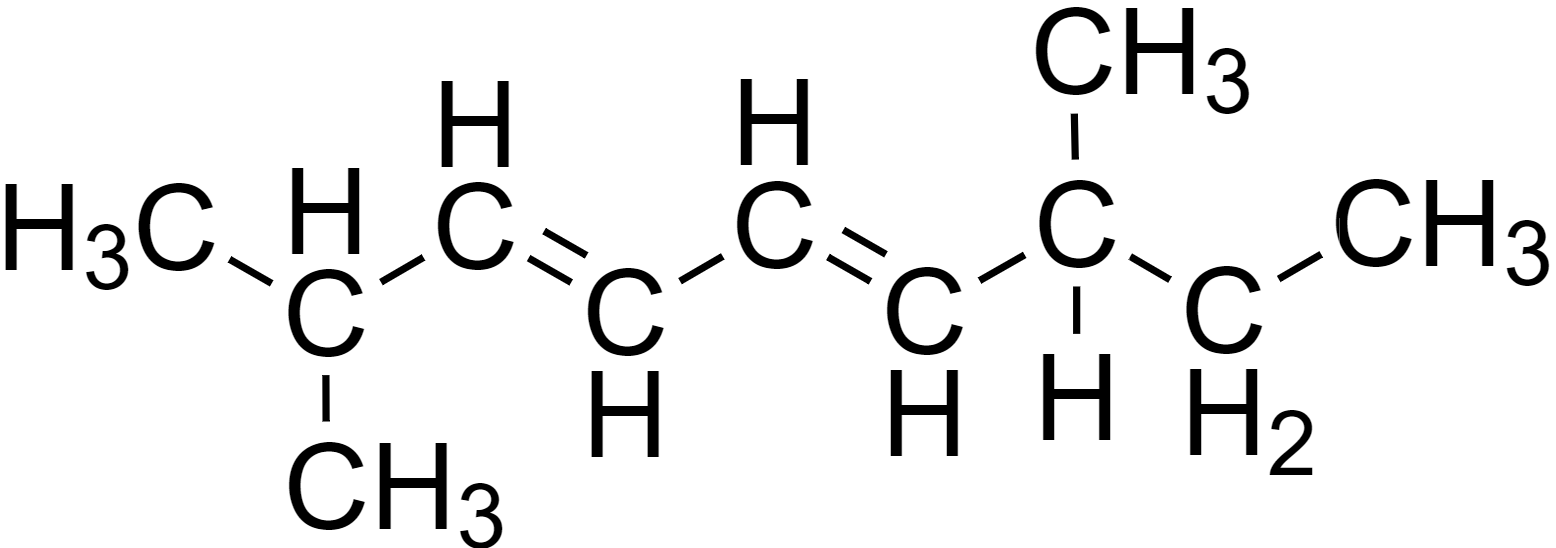
Now, the IUPAC nomenclature of the compound can be determined through the following steps.
1. Determine the parent hydrocarbon chain.
- The longest hydrocarbon chain is the parent hydrocarbon chain.
- In case there are two hydrocarbon chains with the same length, the chain with the most branching is the parent chain.
- The parent chain should have the maximum number of single bonds as well as double bonds.
Here the parent carbon chain is a 9 carbon chain (nona-) containing double bonds.
2. Identify the functional group.
Here there are no functional groups.
3. Identify the side chains.
Here there are two methyl chains.
4. The identification of unsaturated bonds.
Here there are two unsaturated double bonds.
5. Numbering the chain
The chain is numbered such that the chain has the lowest value. The unsaturated bonds and the side chains present must also have the least possible value.
Hence the chain will be numbered from left to right.
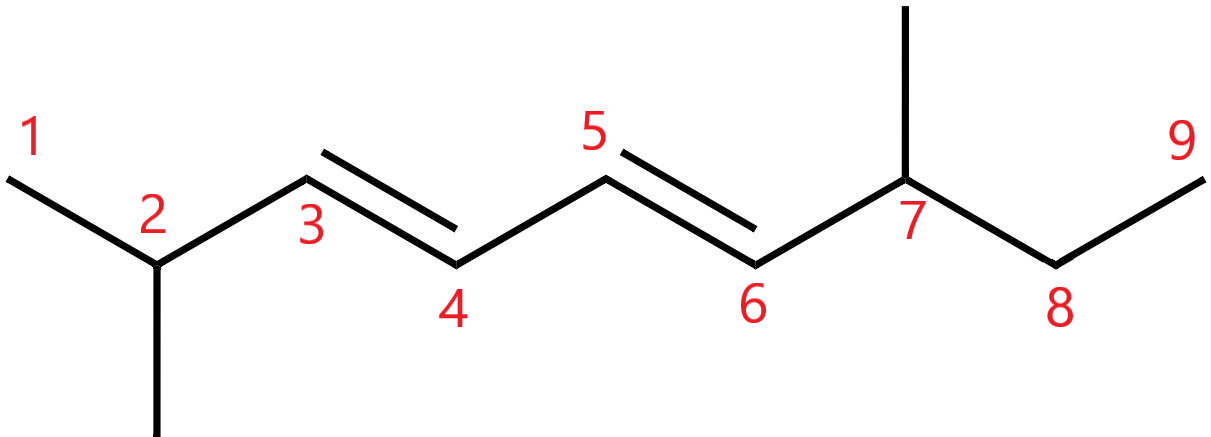
6. When there are multiple substituents of the same type, prefixes like di-, tri-, are added.
So, since there are two methyl groups and two double bonds, we will use dimethyl and diene.
7. Name the compound.
- The numbers are separated by commas and the sets of numbers are separated from words by hyphens (-).
So, the IUPAC name of the compound is option (A) 2,7-dimethyl-3,5-nonadiene.
Note:
It should be noted that while IUPAC nomenclature can help determine the structural formula of a compound, for complex molecules, they are tedious and long and hence are avoided in normal conversation. Simple and common names are used instead.
Complete answer:
Let us first redraw the compound for better clarity.

Now, the IUPAC nomenclature of the compound can be determined through the following steps.
1. Determine the parent hydrocarbon chain.
- The longest hydrocarbon chain is the parent hydrocarbon chain.
- In case there are two hydrocarbon chains with the same length, the chain with the most branching is the parent chain.
- The parent chain should have the maximum number of single bonds as well as double bonds.
Here the parent carbon chain is a 9 carbon chain (nona-) containing double bonds.
2. Identify the functional group.
Here there are no functional groups.
3. Identify the side chains.
Here there are two methyl chains.
4. The identification of unsaturated bonds.
Here there are two unsaturated double bonds.
5. Numbering the chain
The chain is numbered such that the chain has the lowest value. The unsaturated bonds and the side chains present must also have the least possible value.
Hence the chain will be numbered from left to right.

6. When there are multiple substituents of the same type, prefixes like di-, tri-, are added.
So, since there are two methyl groups and two double bonds, we will use dimethyl and diene.
7. Name the compound.
- The numbers are separated by commas and the sets of numbers are separated from words by hyphens (-).
So, the IUPAC name of the compound is option (A) 2,7-dimethyl-3,5-nonadiene.
Note:
It should be noted that while IUPAC nomenclature can help determine the structural formula of a compound, for complex molecules, they are tedious and long and hence are avoided in normal conversation. Simple and common names are used instead.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




