
The IUPAC name of:
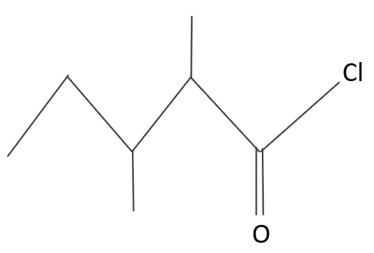
A.$3,4 - $ dimethylpentanoyl chloride
B.$1 - $ chloro-$1 - $ oxo $2,3 - $ dimethyl pentane
C.$2 - $ ethyl $3 - $ methylbutanoyl chloride
D.$2,3 - $ dimethylpentanoyl chloride

Answer
551.7k+ views
Hint: Functional group: In the hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
Complete answer:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
The first step in the IUPAC naming system is to identify the longest carbon chain containing the groups. Here in the given compound the largest chain contains five carbons. So the name of the parent chain will be pentane. Now here one function group is attached to the parent chain i.e. –oyl chloride group which is $ - COCl$ . And two branches are there in which methyl groups are present in the compound. Now we will number the carbon such that the carbon which contains functionality gets lower. So we will do the numbering from the right side. So the carbon containing the -oyl chloride group will get number one and the two branching carbon atoms will get number as two and three. So the IUPAC name will be as: $2,3 - $ methylpentanoyl chloride. Because of the presence of –oyl chloride group in the compound the suffix -oyl chloride is used in the IUPAC name of the molecule.
Hence option C is correct.
Note:
Suffix to some functional groups are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Complete answer:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
| Number of carbon atom in alkane | Name of the parent chain |
| One | Methane |
| Two | Ethane |
| Three | Propane |
| Four | Butane |
| Five | Pentane |
| Six | Hexane |
| Seven | Heptane |
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
The first step in the IUPAC naming system is to identify the longest carbon chain containing the groups. Here in the given compound the largest chain contains five carbons. So the name of the parent chain will be pentane. Now here one function group is attached to the parent chain i.e. –oyl chloride group which is $ - COCl$ . And two branches are there in which methyl groups are present in the compound. Now we will number the carbon such that the carbon which contains functionality gets lower. So we will do the numbering from the right side. So the carbon containing the -oyl chloride group will get number one and the two branching carbon atoms will get number as two and three. So the IUPAC name will be as: $2,3 - $ methylpentanoyl chloride. Because of the presence of –oyl chloride group in the compound the suffix -oyl chloride is used in the IUPAC name of the molecule.
Hence option C is correct.
Note:
Suffix to some functional groups are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




