
The IUPAC name of
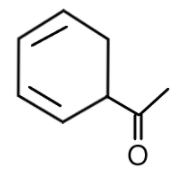
A. $1 - cyclohexa - 2,4 - dienylethanone$
B. $3 - cyclohexa - 2,4 - dienylethanone$
C. $1 - cyclohexa - 3,5 - dienylethanone$
D. $3 - cyclohexa - 3,5 - dienylethanone$

Answer
573.3k+ views
Hint:As we all know, the IUPAC system consists of a root or base, primary and secondary prefix and suffix and any substituent for naming a compound. The common names of these compounds are derived by writing the number of carbons in them and appropriate names of alkyl or aryl groups.
Complete answer:
As we have learnt that IUPAC is the short for International Union of Pure and Applied Chemistry and it provides some rules for naming of the organic compounds. Using these rules we can write a unique name for any distinct compound and likewise we can make the structure from the given IUPAC name. IUPAC system consists of three main features which are: a root representing the chain of carbon atoms in molecular structure, presence of primary prefix, secondary prefix, primary suffix and secondary suffix and lastly the name of the substituents completing the molecular structure or formula.
So let us try naming the given structure with the help of these rules. First step is to find out the parent chain and write the number of carbon, in the given structure we can see that benzene is not the parent molecule but there are two carbons present which will be considered as parent chains here. We see that functional group $ - CO - C{H_3}$, a ketone is present having two carbons so the parent chain will be of two carbons and the root name will be ethanone.
Then, we will number the carbons in the structure, the substituent attached to the functional group is a cyclohexane having bonds at the 2nd and 4th carbons. It is attached at the 1st carbon of ethanone which can be seen in:
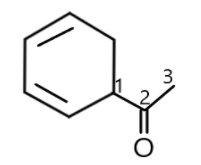
We should know that the name of unsaturated monocyclic hydrocarbons having no side chain are formed by using the prefix ‘cyclo’ before the name of acyclic unsaturated unbranched hydrocarbons having the same number of carbon atoms. So the name will be given as cyclohexane and the carbon with free valence is considered as 1 and the name of univalent radicals derived from unsaturated monocyclic hydrocarbons have the endings dienyl with double bonds at 2nd and 4th carbon.
So, the IUPAC name of the given structure will be: $1 - cyclohexa - 2,4 - dienylethanone$
Hence the correct answer is A. $1 - cyclohexa - 2,4 - dienylethanone$
Note:
Always remember that the parent chain will start from the side of attachment of the functional group. Also, while naming any compound put commas between numbers and dashes between letters and the numbers and while making structures first make the parent chain then add the substituents.
Complete answer:
As we have learnt that IUPAC is the short for International Union of Pure and Applied Chemistry and it provides some rules for naming of the organic compounds. Using these rules we can write a unique name for any distinct compound and likewise we can make the structure from the given IUPAC name. IUPAC system consists of three main features which are: a root representing the chain of carbon atoms in molecular structure, presence of primary prefix, secondary prefix, primary suffix and secondary suffix and lastly the name of the substituents completing the molecular structure or formula.
So let us try naming the given structure with the help of these rules. First step is to find out the parent chain and write the number of carbon, in the given structure we can see that benzene is not the parent molecule but there are two carbons present which will be considered as parent chains here. We see that functional group $ - CO - C{H_3}$, a ketone is present having two carbons so the parent chain will be of two carbons and the root name will be ethanone.
Then, we will number the carbons in the structure, the substituent attached to the functional group is a cyclohexane having bonds at the 2nd and 4th carbons. It is attached at the 1st carbon of ethanone which can be seen in:

We should know that the name of unsaturated monocyclic hydrocarbons having no side chain are formed by using the prefix ‘cyclo’ before the name of acyclic unsaturated unbranched hydrocarbons having the same number of carbon atoms. So the name will be given as cyclohexane and the carbon with free valence is considered as 1 and the name of univalent radicals derived from unsaturated monocyclic hydrocarbons have the endings dienyl with double bonds at 2nd and 4th carbon.
So, the IUPAC name of the given structure will be: $1 - cyclohexa - 2,4 - dienylethanone$
Hence the correct answer is A. $1 - cyclohexa - 2,4 - dienylethanone$
Note:
Always remember that the parent chain will start from the side of attachment of the functional group. Also, while naming any compound put commas between numbers and dashes between letters and the numbers and while making structures first make the parent chain then add the substituents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




