
The IUPAC name is:
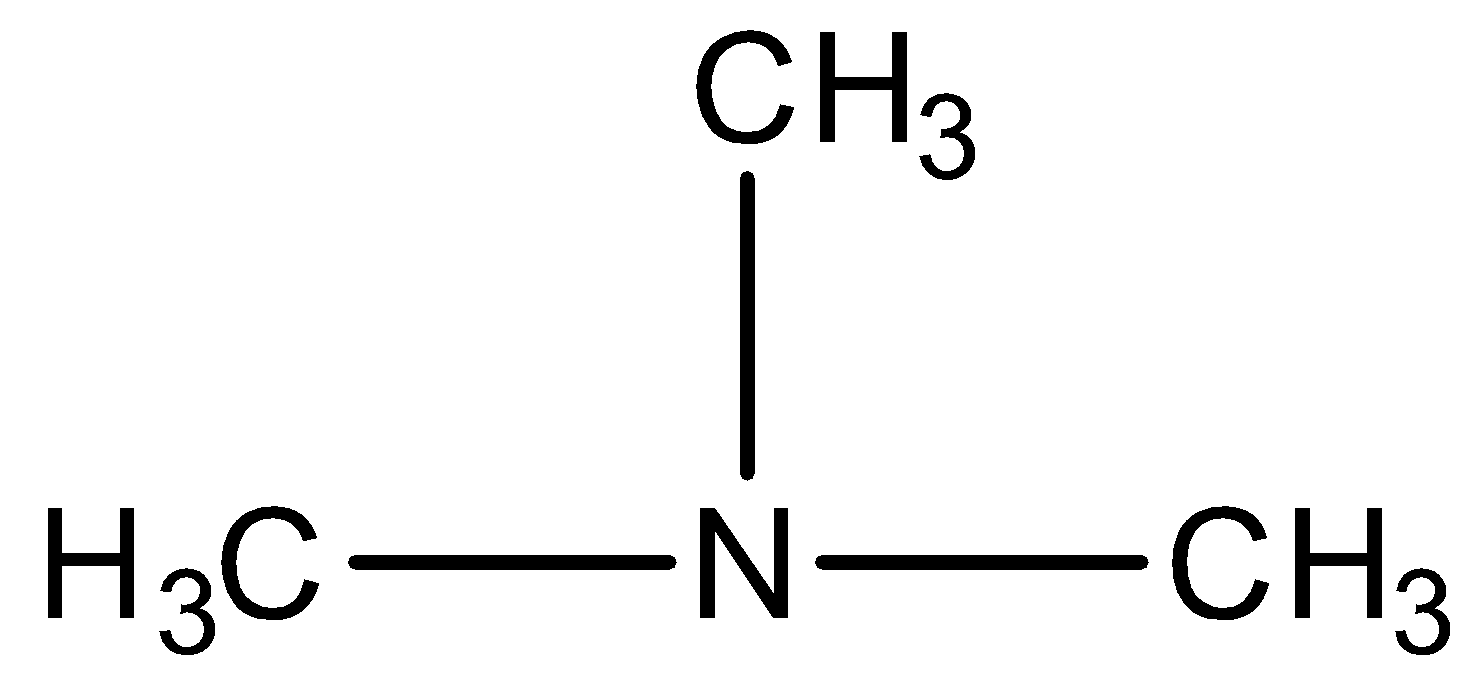
A. Trimethylamine
B. 2-methyl ethanamine
C. N,N-Dimethylmethanamine
D. Trimethyl ammonia

Answer
559.2k+ views
Hint: We know that amines are basic chemical derivatives of ammonia in which the bonded hydrogens are replaced by an alkyl or aryl groups. Amines are classified into,
Primary amine: One organic group attached to nitrogen atoms.
Secondary amine: Two organic groups bonded to nitrogen atoms.
Tertiary amine: Three organic groups bonded to nitrogen atoms.
Complete step by step answer:
The given compound is,
We have to know that all the three hydrogens of ammonia are replaced by alkyl groups and hence it is a tertiary amine (\[3^\circ \] amine).
The IUPAC name of tertiary amine is given by the set of rules given below:
IUPAC nomenclature of tertiary amine:
The parent compound, that is, the longest alkyl chain is identified.
The parent compound is named as alkanamine.
The other two-alkyl groups are named as $N - $ alkyl groups, and they are used as a prefix in front of the parent alkanamine.
If the two alkyl groups are different, they are listed sequentially.
If the two groups are identical, they are written as $N - $ dialkyl.
In the given compound, the longest alkyl chain is methane; therefore the parent compound is methanamine. There are two methyl groups present and they are identical. So, the compound is named as N,N-dimethylmethanamine.
So, the correct answer is Option C.
Note: We can write the molecular formula of trimethylamine as ${C_3}{H_9}N$. We have to know that trimethylamine is colourless gas that has fishlike smell at lower concentrations. They produce harmful oxides of nitrogen when they undergo combustion. The conjugate base of trimethylamine is trimethylammonium.
Primary amine: One organic group attached to nitrogen atoms.
Secondary amine: Two organic groups bonded to nitrogen atoms.
Tertiary amine: Three organic groups bonded to nitrogen atoms.
Complete step by step answer:
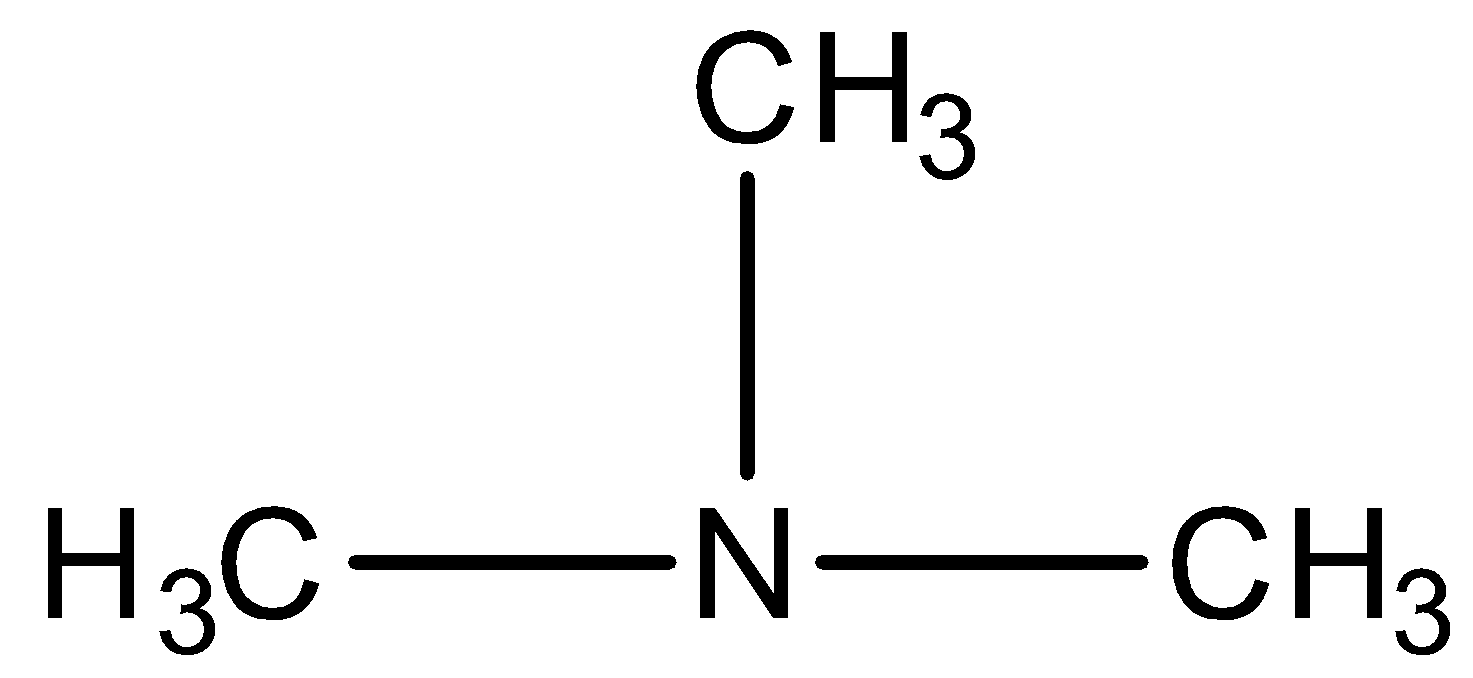
The given compound is,

We have to know that all the three hydrogens of ammonia are replaced by alkyl groups and hence it is a tertiary amine (\[3^\circ \] amine).
The IUPAC name of tertiary amine is given by the set of rules given below:
IUPAC nomenclature of tertiary amine:
The parent compound, that is, the longest alkyl chain is identified.
The parent compound is named as alkanamine.
The other two-alkyl groups are named as $N - $ alkyl groups, and they are used as a prefix in front of the parent alkanamine.
If the two alkyl groups are different, they are listed sequentially.
If the two groups are identical, they are written as $N - $ dialkyl.
In the given compound, the longest alkyl chain is methane; therefore the parent compound is methanamine. There are two methyl groups present and they are identical. So, the compound is named as N,N-dimethylmethanamine.
So, the correct answer is Option C.
Note: We can write the molecular formula of trimethylamine as ${C_3}{H_9}N$. We have to know that trimethylamine is colourless gas that has fishlike smell at lower concentrations. They produce harmful oxides of nitrogen when they undergo combustion. The conjugate base of trimethylamine is trimethylammonium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




