
The ${\text{IUPAC}}$ name for the hydrocarbon represented by:
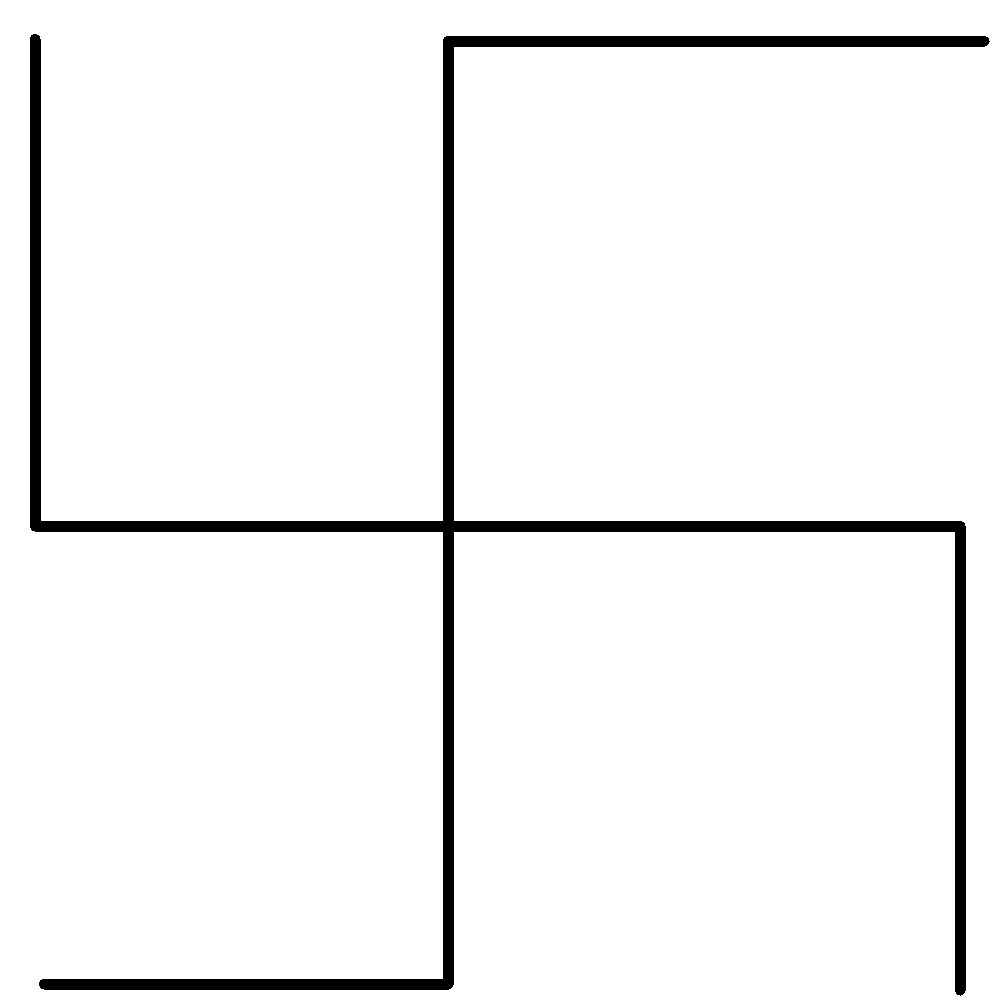
A) $3,3 - $diethylpentane
B) $2 - $ethylpentane
C) tetraethyl carbon
D) neo-nonane

Answer
585k+ views
Hint: Follow the rules of naming a compound according to ${\text{IUPAC}}$ system. One can also find out the name by using the number of molecules attached and the longest chain present in the structure and choose the correct option.
Complete step by step answer:
1) First of all let us learn about how a ${\text{IUPAC}}$ nomenclature is given to a compound by rules and applying those rules by keeping in mind the structure given in the question. The ${\text{IUPAC}}$ nomenclature is given as per the rules which are described below,
2) Rule ${\text{1}}$ - Select the longest carbon chain present in the structure in this case which is $C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_3}$ which is pentane.
3) Rule ${\text{2}}$ - Name the carbon atoms giving first priority to the carbon which is attached to a functional group or has the nearest functional group which in this case there is $C{H_2} - C{H_3}$ i.e. ethane group is present which is attached to the longest chain. The two groups $C{H_2} - C{H_3}$ are attached in the center of the chain hence, we can give numbering from any side of the chain.
4) Rule ${\text{3}}$ - Number the functional groups present in the structure other than the longest carbon chain accordingly and write them while naming as per their number which in this case $C{H_2} - C{H_3}$ are given number ${\text{3}}$ and the group is named as ethyl in ${\text{IUPAC}}$ nomenclature.
5) Rule ${\text{4}}$ - The longest carbon chain is the basic chain and the name of the compound is based on this which in this case is a pentane chain that has five carbon atoms and is named at last.
6) Therefore, the ${\text{IUPAC}}$ name of the compound will be $3,3 - $diethylpentane
So, the correct answer is Option A .
Note:
The solubility of a substance is dependent on the nature of the solute and solvent. Solubility also depends on physical factors such as temperature and pressure. As the solubility is the number of grams dissolved per number of grams it doesn’t show any unit. The solubility concept should not be confused with the ability of a solution to dissolve a substance as both are different aspects.
Complete step by step answer:
1) First of all let us learn about how a ${\text{IUPAC}}$ nomenclature is given to a compound by rules and applying those rules by keeping in mind the structure given in the question. The ${\text{IUPAC}}$ nomenclature is given as per the rules which are described below,
2) Rule ${\text{1}}$ - Select the longest carbon chain present in the structure in this case which is $C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_3}$ which is pentane.
3) Rule ${\text{2}}$ - Name the carbon atoms giving first priority to the carbon which is attached to a functional group or has the nearest functional group which in this case there is $C{H_2} - C{H_3}$ i.e. ethane group is present which is attached to the longest chain. The two groups $C{H_2} - C{H_3}$ are attached in the center of the chain hence, we can give numbering from any side of the chain.
4) Rule ${\text{3}}$ - Number the functional groups present in the structure other than the longest carbon chain accordingly and write them while naming as per their number which in this case $C{H_2} - C{H_3}$ are given number ${\text{3}}$ and the group is named as ethyl in ${\text{IUPAC}}$ nomenclature.
5) Rule ${\text{4}}$ - The longest carbon chain is the basic chain and the name of the compound is based on this which in this case is a pentane chain that has five carbon atoms and is named at last.
6) Therefore, the ${\text{IUPAC}}$ name of the compound will be $3,3 - $diethylpentane
So, the correct answer is Option A .
Note:
The solubility of a substance is dependent on the nature of the solute and solvent. Solubility also depends on physical factors such as temperature and pressure. As the solubility is the number of grams dissolved per number of grams it doesn’t show any unit. The solubility concept should not be confused with the ability of a solution to dissolve a substance as both are different aspects.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




