
The hydrolysis of 2-bromo-3-methyl butane by ${{\text{S}}_{\text{N}}}\text{1}$ mechanism gives mainly:
A. 3-Methyl-2-butanol
B. 2-Methyl-2-butanol
C. 2,2-Dimethyl-1-propanol
D. 2-Methyl-1-butanol
Answer
598.5k+ views
Hint: Hydrolysis means the reaction of compounds with water. ${{\text{S}}_{\text{N}}}\text{1}$ mechanism mainly follows two to three steps, formation of carbocation, followed by shifting of positive charge and then deprotonation of nucleophile. It is a nucleophilic substitution reaction.
Complete step by step answer:
Let us find the final product using ${{\text{S}}_{\text{N}}}\text{1}$ mechanism:
Step (1)- The carbon-bromine bond is polar because of the difference in electronegativity of the elements. Bromine is more polar than carbon. So, the bond breaks to form the carbocation and the leaving group will be bromide ion.
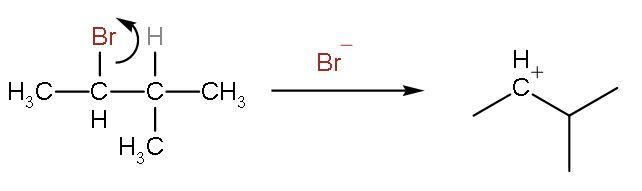
Step (2)- The positive charge on the carbon is shifted as there can be formation of more stable carbocation. The stability of a carbocation seen by hyperconjugation effect. The more the number of hyper conjugating structures the more will be the stability of carbocation. This carbocation has 4 $\alpha -$hydrogens. Hyper conjugating structures are counted by counting the number of $\alpha -$hydrogens. So, there will be hydride shift or shifting of hydrogen atom.
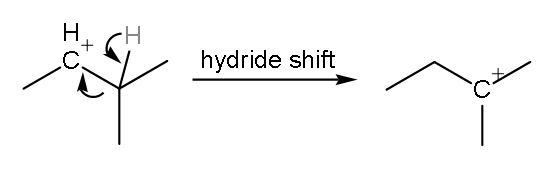
This is more stable as it has 6 $\alpha -$hydrogens.
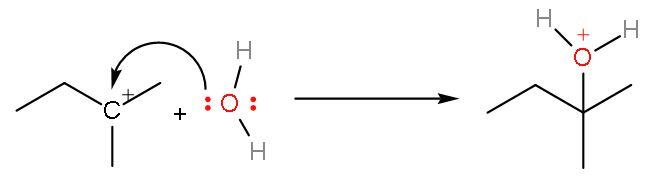
Step (3)- Attack of nucleophile that is water on the carbocation, so, oxonium ion is formed. There will be positive signs on electronegative oxygen atoms.
Step (4)- The positive charge on the oxygen atom is removed as ${{\text{H}}^{+}}$ ion gets removed from the attached water molecule. The final product formed will be 2-methyl-2-butanol.
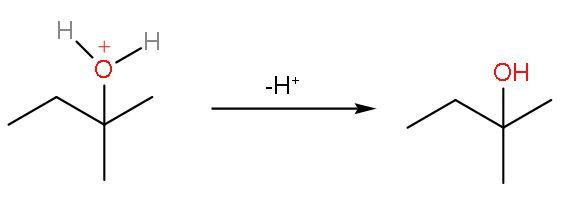
The correct answer is option ‘b’, 2-methyl-2-butanol.
So, the correct answer is “Option B”.
Note: The role of solvent is very important for ${{\text{S}}_{\text{N}}}\text{1}$ reaction. The preferred solvents for ${{\text{S}}_{\text{N}}}\text{1}$ reaction are both polar and protic solvents. The polar nature of the solvent helps in stabilizing the ionic intermediates formed whereas the protic nature of the solvent helps to solvate the leaving group.
Complete step by step answer:
Let us find the final product using ${{\text{S}}_{\text{N}}}\text{1}$ mechanism:
Step (1)- The carbon-bromine bond is polar because of the difference in electronegativity of the elements. Bromine is more polar than carbon. So, the bond breaks to form the carbocation and the leaving group will be bromide ion.

Step (2)- The positive charge on the carbon is shifted as there can be formation of more stable carbocation. The stability of a carbocation seen by hyperconjugation effect. The more the number of hyper conjugating structures the more will be the stability of carbocation. This carbocation has 4 $\alpha -$hydrogens. Hyper conjugating structures are counted by counting the number of $\alpha -$hydrogens. So, there will be hydride shift or shifting of hydrogen atom.

This is more stable as it has 6 $\alpha -$hydrogens.
Step (3)- Attack of nucleophile that is water on the carbocation, so, oxonium ion is formed. There will be positive signs on electronegative oxygen atoms.

Step (4)- The positive charge on the oxygen atom is removed as ${{\text{H}}^{+}}$ ion gets removed from the attached water molecule. The final product formed will be 2-methyl-2-butanol.

The correct answer is option ‘b’, 2-methyl-2-butanol.
So, the correct answer is “Option B”.
Note: The role of solvent is very important for ${{\text{S}}_{\text{N}}}\text{1}$ reaction. The preferred solvents for ${{\text{S}}_{\text{N}}}\text{1}$ reaction are both polar and protic solvents. The polar nature of the solvent helps in stabilizing the ionic intermediates formed whereas the protic nature of the solvent helps to solvate the leaving group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




