
The hybridization state of the functional carbon changes from:
$C{H_3}CN\xrightarrow[{heat}]{{{H_3}{O^ + }}}C{H_3}COOH$
A. $s{p^3}$ to $s{p^2}$
B. $s{p^2}$to $s{p^3}$
C. sp to $s{p^2}$
D. $s{p^2}$ to sp
Answer
569.1k+ views
Hint: In methyl cyanide the functional group attached is cyanide. In cyanide the carbon is attached to nitrogen by three bonds. In ethanoic acid, the functional group present is carboxylic acid where the carbon is attached to one oxygen by double bond and with one hydroxyl group by single bond.
Complete step by step answer:
In the given reaction, methyl cyanide on heating in water gives ethanoic acid.
In methyl cyanide, the functional group is cyanide and a methyl group is attached to the cyanide group.
The structure of methyl cyanide is shown below.
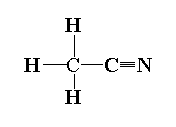
In methyl cyanide, the first carbon is $s{p^3}$ hybridized as four sigma bonds is present and the second carbon of the functional group is sp hybridized as two pi and one sigma bond is present.
In ethanoic acid, the functional group is carboxylic acid, where the methyl group is attached to the carboxylic acid functional group. In the carboxylic acid functional group, the carbon is attached to one oxygen atom by a double bond and by a hydroxyl group by a single bond.
The structure of ethanoic acid is shown below.
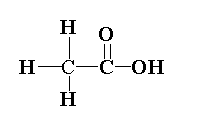
In ethanoic acid, the first carbon is $s{p^3}$ hybridized as four sigma bonds is present and the second carbon of the functional group is $s{p^2}$ hybridized as two sigma and one pi bond is present.
Thus, in the given reaction, the hybridization of carbon of cyanide functional group which is sp changes to $s{p^2}$, which is the hybridization of carbon of carboxylic acid functional group.
So, the correct answer is Option C.
Note: In ethanoic acid and methyl cyanide, the carbon-carbon bond is formed by the overlap of both the hybridization of carbon. In the Hybridization Atomic orbitals combine to form new hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory.
Complete step by step answer:
In the given reaction, methyl cyanide on heating in water gives ethanoic acid.
In methyl cyanide, the functional group is cyanide and a methyl group is attached to the cyanide group.
The structure of methyl cyanide is shown below.

In methyl cyanide, the first carbon is $s{p^3}$ hybridized as four sigma bonds is present and the second carbon of the functional group is sp hybridized as two pi and one sigma bond is present.
In ethanoic acid, the functional group is carboxylic acid, where the methyl group is attached to the carboxylic acid functional group. In the carboxylic acid functional group, the carbon is attached to one oxygen atom by a double bond and by a hydroxyl group by a single bond.
The structure of ethanoic acid is shown below.

In ethanoic acid, the first carbon is $s{p^3}$ hybridized as four sigma bonds is present and the second carbon of the functional group is $s{p^2}$ hybridized as two sigma and one pi bond is present.
Thus, in the given reaction, the hybridization of carbon of cyanide functional group which is sp changes to $s{p^2}$, which is the hybridization of carbon of carboxylic acid functional group.
So, the correct answer is Option C.
Note: In ethanoic acid and methyl cyanide, the carbon-carbon bond is formed by the overlap of both the hybridization of carbon. In the Hybridization Atomic orbitals combine to form new hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




