
The hybridization of the first and second carbon atoms in propadiene respectively is:
A. $sp,s{{p}^{2}}$
B. $s{{p}^{2}},sp$
C. $s{{p}^{2}},s{{p}^{2}}$
D. $sp,sp$
Answer
577.5k+ views
Hint: Visualize the structure of propadiene and then consider the single and double bonds that may be present between each of the carbon atoms. Label the carbon atoms and then determine the hybridization.
Complete answer:
First, we will look at the structure of propadiene so that we will get an idea about the hybridization of the carbon atoms based on any single, double, and triple bonds that are present in the molecule. The structure of propadiene is as follows.
\[{{H}_{2}}{{C}_{1}}={{C}^{2}}={{C}_{3}}{{H}_{2}}\]
Here, we can see that there are two double bonds present between all three carbon atoms. The carbon atoms are labeled from left to right. We have to find the hybridization of the first and the second carbon in this molecule
We can determine the hybridization of each atom based on the type of bonds and the number of sigma and pi bonds that it forms with other atoms.
- For the first atom, we can see that it forms 3 sigma bonds, 2 of them are formed with 2 hydrogen atoms and one of them is with the adjacent carbon atom. We can see the double bond present between the first and the second carbon atom, the second bond is a pi bond that involves the sideways overlap of the hybridized orbitals. It needs only one orbital for the sideways overlap and hence it will be $s{{p}^{2}}$ hybridized.
- For the second atom, we can see that it forms 2 sigma bonds, each with the adjacent carbon atoms 1 and 3. It forms 2 pi bonds with the same carbon atoms in the form of double bonds. So, it will require two orbitals that are able to perform the sideways overlap that forms the pi bond. Hence, the second carbon atom will be $sp$ hybridized.
The diagrammatic representation of this is:
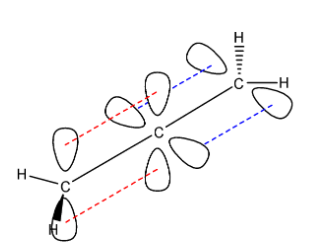
Here, we can see that the solid black bond between the carbon atoms is the sigma bond. The dashed red lines form the pi bond between the first and the second carbon atom, and the dashed blue lines form the pi bond between the second and the third carbon atom.
In the first carbon atom, the angle between the sigma bonds present is $120{}^\circ $ so we can say that it is $s{{p}^{2}}$ hybridized due to the trigonal planar arrangement of the molecular orbitals. Both the terminal carbon atoms have the same hybridization of $s{{p}^{2}}$. The central carbon atom has linear sigma bonds with the adjacent carbon atoms, hence we can say that the central atom is $sp$ hybridized.
Hence, the answer to this question is ‘B. $s{{p}^{2}},sp$’.
Note:
Remember that although the central carbon atom has only double bonds involved, it is $sp$ hybridized. We should always consider the number of pi bonds that the carbon atom is forming and not just the type of bonds that it is forming.
Complete answer:
First, we will look at the structure of propadiene so that we will get an idea about the hybridization of the carbon atoms based on any single, double, and triple bonds that are present in the molecule. The structure of propadiene is as follows.
\[{{H}_{2}}{{C}_{1}}={{C}^{2}}={{C}_{3}}{{H}_{2}}\]
Here, we can see that there are two double bonds present between all three carbon atoms. The carbon atoms are labeled from left to right. We have to find the hybridization of the first and the second carbon in this molecule
We can determine the hybridization of each atom based on the type of bonds and the number of sigma and pi bonds that it forms with other atoms.
- For the first atom, we can see that it forms 3 sigma bonds, 2 of them are formed with 2 hydrogen atoms and one of them is with the adjacent carbon atom. We can see the double bond present between the first and the second carbon atom, the second bond is a pi bond that involves the sideways overlap of the hybridized orbitals. It needs only one orbital for the sideways overlap and hence it will be $s{{p}^{2}}$ hybridized.
- For the second atom, we can see that it forms 2 sigma bonds, each with the adjacent carbon atoms 1 and 3. It forms 2 pi bonds with the same carbon atoms in the form of double bonds. So, it will require two orbitals that are able to perform the sideways overlap that forms the pi bond. Hence, the second carbon atom will be $sp$ hybridized.
The diagrammatic representation of this is:

Here, we can see that the solid black bond between the carbon atoms is the sigma bond. The dashed red lines form the pi bond between the first and the second carbon atom, and the dashed blue lines form the pi bond between the second and the third carbon atom.
In the first carbon atom, the angle between the sigma bonds present is $120{}^\circ $ so we can say that it is $s{{p}^{2}}$ hybridized due to the trigonal planar arrangement of the molecular orbitals. Both the terminal carbon atoms have the same hybridization of $s{{p}^{2}}$. The central carbon atom has linear sigma bonds with the adjacent carbon atoms, hence we can say that the central atom is $sp$ hybridized.
Hence, the answer to this question is ‘B. $s{{p}^{2}},sp$’.
Note:
Remember that although the central carbon atom has only double bonds involved, it is $sp$ hybridized. We should always consider the number of pi bonds that the carbon atom is forming and not just the type of bonds that it is forming.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




