
The hybridization of phosphorus in $POC{l_3}$ is the same as in:
A.P in $PC{l_3}$
B.S in $S{F_4}$
C.Cl in $Cl{F_3}$
D.B in $BC{l_3}$
Answer
574.5k+ views
Hint:Hybridization is defined as the intermixing of orbitals to form new hybrid orbitals. It is basically a concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics.
Complete step by step solution:
During the process of hybridization, the atomic orbitals of similar energy are mixed together to form new hybrid orbitals. It happens only during the bond formation and not in an isolated gaseous atom. Moreover, the shape of the molecule can be predicted if the hybridization of the molecule is known. There are different types of hybridization such as $s{p^3},s{p^2},s{p^3}{d^2},s{p^3}{d^3}$ and many more.
Now, in case of $POC{l_3}$ and in case of $PC{l_3}$, P atom is $s{p^3}$ hybridized. In $PC{l_3}$, P atom has three bond pair of electrons and one lone pair of electrons whereas in case of $POC{l_3}$, P atom has for bonding domains which include one $P = O$ double bond. Both the structures are as shown:
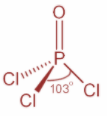
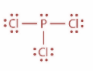
Now, when one ‘s’ orbital and three ‘p’ orbitals belong to the same shell of an atom mix together to form four new equivalent orbitals then this type of hybridization is known as tetrahedral hybridization or $s{p^3}$ hybridization. Example methane.
Hence, option A is correct.
Note:
Don’t get confused with molecular and hybrid orbitals. The interaction between the atomic orbitals of two different atoms result in molecular orbitals whereas when atomic orbitals of the same atom interact, then they form hybrid orbitals.
Complete step by step solution:
During the process of hybridization, the atomic orbitals of similar energy are mixed together to form new hybrid orbitals. It happens only during the bond formation and not in an isolated gaseous atom. Moreover, the shape of the molecule can be predicted if the hybridization of the molecule is known. There are different types of hybridization such as $s{p^3},s{p^2},s{p^3}{d^2},s{p^3}{d^3}$ and many more.
Now, in case of $POC{l_3}$ and in case of $PC{l_3}$, P atom is $s{p^3}$ hybridized. In $PC{l_3}$, P atom has three bond pair of electrons and one lone pair of electrons whereas in case of $POC{l_3}$, P atom has for bonding domains which include one $P = O$ double bond. Both the structures are as shown:

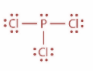
Now, when one ‘s’ orbital and three ‘p’ orbitals belong to the same shell of an atom mix together to form four new equivalent orbitals then this type of hybridization is known as tetrahedral hybridization or $s{p^3}$ hybridization. Example methane.
Hence, option A is correct.
Note:
Don’t get confused with molecular and hybrid orbitals. The interaction between the atomic orbitals of two different atoms result in molecular orbitals whereas when atomic orbitals of the same atom interact, then they form hybrid orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




