
The hybridization of $N,C$ and $O$ shown in the following compound respectively are:
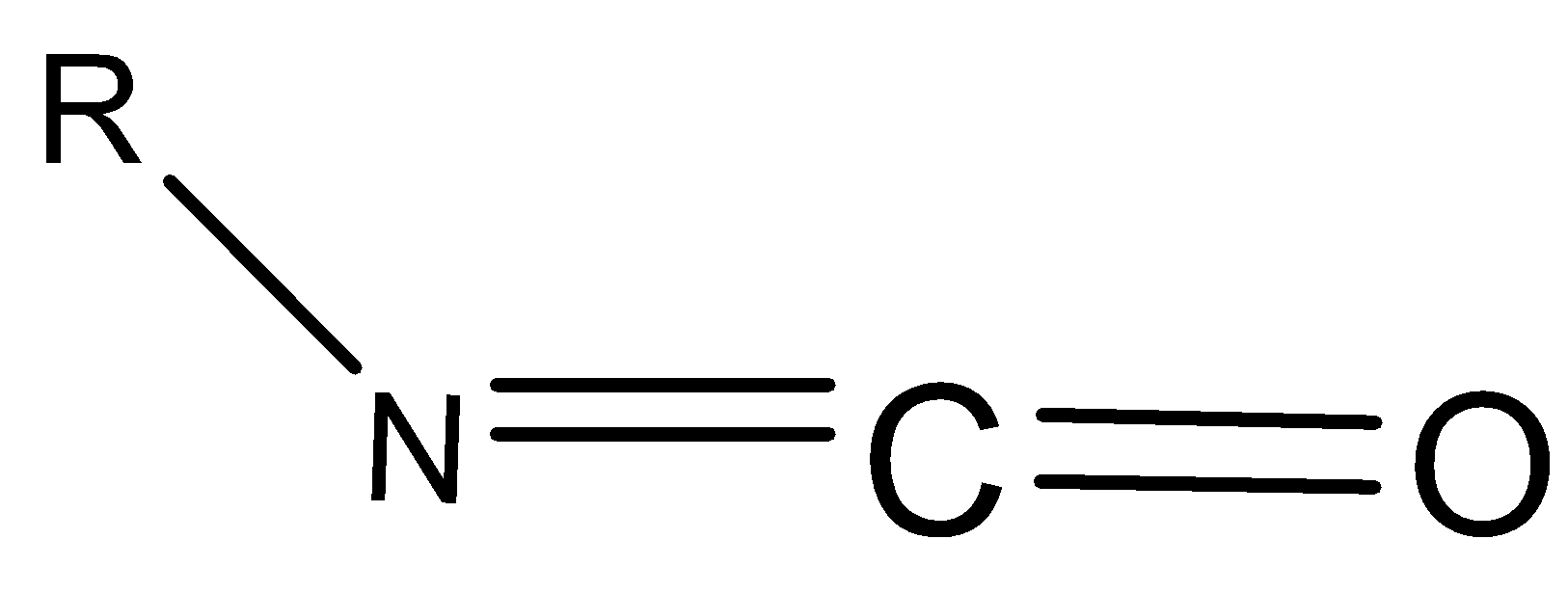
A.$s{p^2},sp,s{p^2}$
B.$s{p^2},s{p^2},s{p^2}$
C.$s{p^2},sp,sp$
D.$sp,sp,s{p^2}$
Answer
478.5k+ views
Hint: Hybridization is characterized as the concept of mixing two atomic orbitals to give a degenerated new form of orbitals with the same energy levels. The combining of atomic orbitals to form new orbitals of different energies and shapes than the initial orbitals is hybridization.
Complete answer:
Orbital hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., then the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Hybridisation ${N_\sigma } + {N_{L.p.}}$
$sp$ $\,\,\,\,$ $2$
$s{p^2}$ $\,\,\,\,$ $3$
$s{p^3}$ $\,\,\,\,$ $4$
$s{p^3}d$ $\,\,\,\,$ $5$
${N_\sigma } + {N_{L.p.}} = 3,2,3$ for $N,C$ and $O$ respectively.
So, the given compound has $s{p^2},so,,s{p^2}$hybridization for $N,C,$ and $O$ atoms respectively.
So, the correct answer is (A) $s{p^2},sp,s{p^2}$
Additional information:
Hybridization of $s$ and $p$ orbitals to form effective $s{p^X}$ hybrids requires that they have comparable radial extent. While $2p$ orbitals are on average less than $10\% $ larger than $2s$, in part attributable to the lack of a radial node in $2p$ orbitals, $3p$ orbitals which have one radial node, exceed the $3s$ orbitals by $20 - 33\% $.The difference in the extent of s and p orbitals increases further down a group. The hybridisation of atoms in chemical bonds can be analyzed by considering localized molecular orbitals.
Note:
Hybridisation helps to explain molecule shape, since the angles between bonds are approximately equal to the angles between hybrid orbitals. In contrast to valence shell electron-pair repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories.
Complete answer:
Orbital hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., then the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Hybridisation ${N_\sigma } + {N_{L.p.}}$
$sp$ $\,\,\,\,$ $2$
$s{p^2}$ $\,\,\,\,$ $3$
$s{p^3}$ $\,\,\,\,$ $4$
$s{p^3}d$ $\,\,\,\,$ $5$
${N_\sigma } + {N_{L.p.}} = 3,2,3$ for $N,C$ and $O$ respectively.
So, the given compound has $s{p^2},so,,s{p^2}$hybridization for $N,C,$ and $O$ atoms respectively.
So, the correct answer is (A) $s{p^2},sp,s{p^2}$
Additional information:
Hybridization of $s$ and $p$ orbitals to form effective $s{p^X}$ hybrids requires that they have comparable radial extent. While $2p$ orbitals are on average less than $10\% $ larger than $2s$, in part attributable to the lack of a radial node in $2p$ orbitals, $3p$ orbitals which have one radial node, exceed the $3s$ orbitals by $20 - 33\% $.The difference in the extent of s and p orbitals increases further down a group. The hybridisation of atoms in chemical bonds can be analyzed by considering localized molecular orbitals.
Note:
Hybridisation helps to explain molecule shape, since the angles between bonds are approximately equal to the angles between hybrid orbitals. In contrast to valence shell electron-pair repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




