
The hybridization of C atoms in tetracyanoethylene is
a.) \[sp,s{{p}^{2}}\]
b.) \[s{{p}^{3}},sp\]
c.) \[s{{p}^{3}},s{{p}^{3}}\]
d.) \[s{{p}^{3}},s{{p}^{2}}\]
Answer
596.1k+ views
Hint: There are a total of five carbons in the given compound. One type of carbon comes from the ‘methane’ part of the compound and another carbon comes from the ‘cyano’ group. There are four cyano carbons in the compound.
Complete step by step solution:
As the name suggests, tetracyanomethane is made up of four ‘cyano’ groups attached to methane. The structure of tetracyanoethylene can be drawn as –
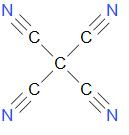
As we can see in the structure, there are two types of carbon in this compound.
One carbon is from the methane group, which is bonded to four carbons instead of hydrogen.
Another carbon is from the nitrile or cyano group, which is bonded to carbon of methane with a single bond and is triple bonded to nitrogen.
Let the two types of carbon be type 1 and type 2 respectively –
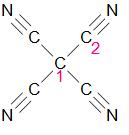
Hybridization of carbon 1 –
As we can see, carbon 1 forms a total of four single bonds.
It has 4 sigma bonds.
Therefore, its hybridization is \[s{{p}^{3}}\].
Hybridization of carbon 2 –
As we can see, carbon 2 forms a total of two types of bonds – one single and one triple bond.
It has two sigma bonds.
Therefore, its hybridization is \[sp\].
Therefore, the answer is – option (b) – \[s{{p}^{3}},sp\].
Additional Information: Hybridization of all atoms of the same type is similar. For example – all carbons of the cyano group are \[sp\] hybridized.
Note: Hybridization is defined as, “the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc.) suitable for the pairing of electrons to form chemical bonds in valence bond theory”. Hybridization can be calculated by the formula:
z = Number of sigma bond + Number of Lone Pairs in Central Metal atom.
Complete step by step solution:
As the name suggests, tetracyanomethane is made up of four ‘cyano’ groups attached to methane. The structure of tetracyanoethylene can be drawn as –

As we can see in the structure, there are two types of carbon in this compound.
One carbon is from the methane group, which is bonded to four carbons instead of hydrogen.
Another carbon is from the nitrile or cyano group, which is bonded to carbon of methane with a single bond and is triple bonded to nitrogen.
Let the two types of carbon be type 1 and type 2 respectively –

Hybridization of carbon 1 –
As we can see, carbon 1 forms a total of four single bonds.
It has 4 sigma bonds.
Therefore, its hybridization is \[s{{p}^{3}}\].
Hybridization of carbon 2 –
As we can see, carbon 2 forms a total of two types of bonds – one single and one triple bond.
It has two sigma bonds.
Therefore, its hybridization is \[sp\].
Therefore, the answer is – option (b) – \[s{{p}^{3}},sp\].
Additional Information: Hybridization of all atoms of the same type is similar. For example – all carbons of the cyano group are \[sp\] hybridized.
Note: Hybridization is defined as, “the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc.) suitable for the pairing of electrons to form chemical bonds in valence bond theory”. Hybridization can be calculated by the formula:
z = Number of sigma bond + Number of Lone Pairs in Central Metal atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




