
The hybridization of \[BeC{l_2}\] in solid state and above 1200 K is respectively
A. \[s{p^3}\], \[s{p^3}\]
B. \[s{p^2}\], \[s{p^2}\]
C. \[s{p^3}\], \[s{p^2}\]
D. \[s{p^3}\], sp
Answer
578.1k+ views
Hint: To solve this question, we need to first understand the method to calculate the hybridization state of any given compound. Then we need to draw and analyse the molecular of the given compounds to determine their hybridization state.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
To determine the hybridization and shape of the given molecule, we must follow these steps:
1.Draw the Lewis structure to understand the rough structure of the given molecule and its bonding pattern. We can use the valence electron concept to make the Lewis structure.
2.After that, we must calculate the number of sigma bonds in the given compound. Sigma bonds can be understood as the single bonds which form the basic skeleton while bonding.
3.Following which, we must calculate the number of lone pairs. We can either count the number of lone pairs present in the Lewis structure or we can calculate the number of lone pairs using the formula: \[\dfrac{{v - b - c}}{2}\], where v is the number of valence electrons, b is the total number of bonds and c is the charge on the atom.
4.We must now calculate the steric number. The formula for steric number is:
Steric number = (number of sigma bonds) + (number of lone pairs)
5.Now, the final step includes to correlate the steric number calculated to the following chart:
Now, the shape of the \[BeC{l_2}\] molecule changes a bit from its solid state to its state at 1200 K. These two molecular structures can be given as:
a)In solid state:
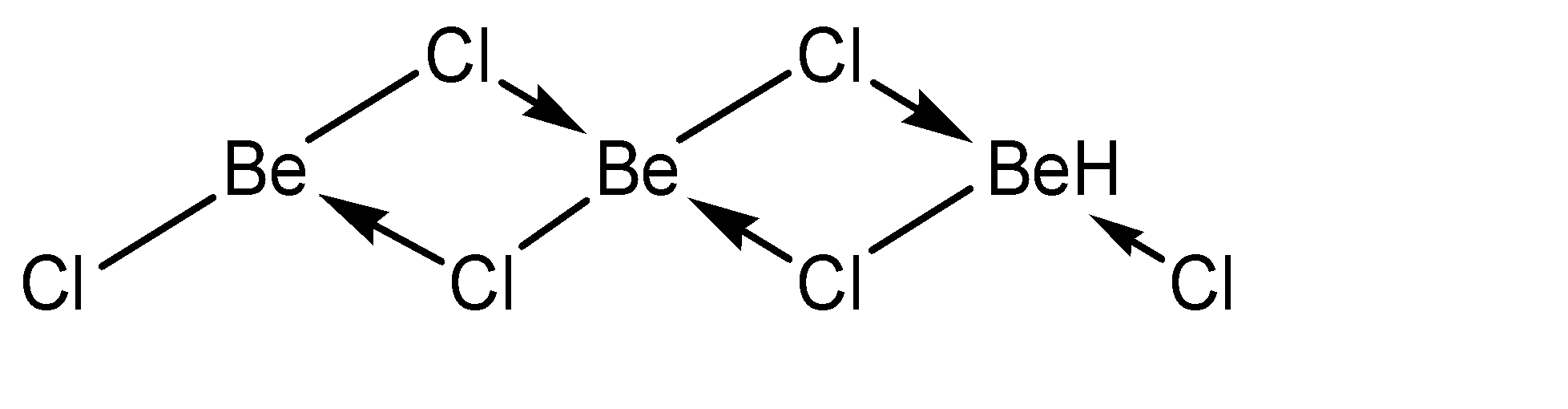
Hence, the number of bonds formed by Be are 4 and there are no lone pairs present. Hence, the hybridization state in this case is \[s{p^3}\]
b)At 1200 K:
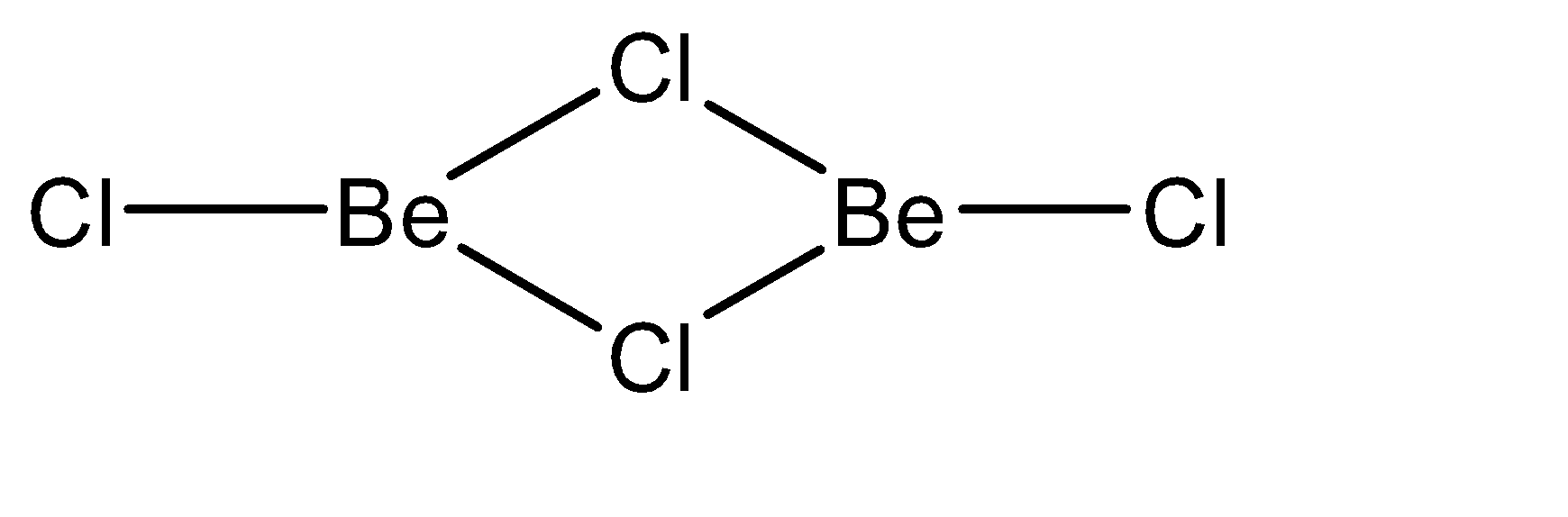
At this temperature, the polymeric structure of beryllium dichloride breaks to form a dimeric structure. In this case, the number of bonds formed are 3. Hence, the hybridization is \[s{p^2}\]
Hence, Option C is the correct option
Note: Hybridization allows for the most stable (and most desirable) structure. When there are hybrid orbitals there are enough electrons to complete the necessary bonds - regardless of whether there is a suitable number of valence electrons.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
To determine the hybridization and shape of the given molecule, we must follow these steps:
1.Draw the Lewis structure to understand the rough structure of the given molecule and its bonding pattern. We can use the valence electron concept to make the Lewis structure.
2.After that, we must calculate the number of sigma bonds in the given compound. Sigma bonds can be understood as the single bonds which form the basic skeleton while bonding.
3.Following which, we must calculate the number of lone pairs. We can either count the number of lone pairs present in the Lewis structure or we can calculate the number of lone pairs using the formula: \[\dfrac{{v - b - c}}{2}\], where v is the number of valence electrons, b is the total number of bonds and c is the charge on the atom.
4.We must now calculate the steric number. The formula for steric number is:
Steric number = (number of sigma bonds) + (number of lone pairs)
5.Now, the final step includes to correlate the steric number calculated to the following chart:
| Steric number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | \[s{p^2}\] | Trigonal planar |
| 4 | \[s{p^3}\] | Tetrahedral |
| 5 | \[s{p^3}d\] | Trigonal bipyramidal |
| 6 | \[s{p^3}{d^2}\] | Octahedral |
| 7 | \[s{p^3}{d^3}\] | Pentagonal bipyramidal |
Now, the shape of the \[BeC{l_2}\] molecule changes a bit from its solid state to its state at 1200 K. These two molecular structures can be given as:
a)In solid state:

Hence, the number of bonds formed by Be are 4 and there are no lone pairs present. Hence, the hybridization state in this case is \[s{p^3}\]
b)At 1200 K:

At this temperature, the polymeric structure of beryllium dichloride breaks to form a dimeric structure. In this case, the number of bonds formed are 3. Hence, the hybridization is \[s{p^2}\]
Hence, Option C is the correct option
Note: Hybridization allows for the most stable (and most desirable) structure. When there are hybrid orbitals there are enough electrons to complete the necessary bonds - regardless of whether there is a suitable number of valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




