
The hybridisation sulphur in \[S{{F}_{4}}\] is:
a.) \[s{{p}^{3}}\]
b.) \[s{{p}^{2}}\]
c.) \[s{{p}^{3}}d\]
d.) \[s{{p}^{3}}{{d}^{2}}\]
Answer
595.5k+ views
Hint: To answer this question, we should know that we use Lewis dot structures to determine bonding patterns in molecules. We should determine the hybridization of any atoms with lone pairs. Lone pairs occupy the hybridized orbitals.
Complete step by step solution:
To answer the hybridisation of sulphur in sulfur tetrafluoride, we have to first understand its Lewis structure and the number of valence electrons that are present. The \[S{{F}_{4}}\] molecule consists of a total of 34 valence electrons. Here 6 will come from sulfur and each of the four fluorine atoms will have 7 electrons.
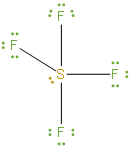
The above structure is the Lewis dot structure of \[S{{F}_{4}}\].
We should note that during the formation of \[S{{F}_{4}}\], the sulphur atom will form bonds with each of fluorine atoms in which 8 of valence electrons are used. And we can say that, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. And also we can say that two electrons will be kept as lone pairs in the sulphur atom. Now we can determine sulphur’s hybridization by taking a count of the number of regions of electron density. When bonding takes place there is a formation of 4 single bonds in sulphur and it has 1 lone pair. Looking at this, we can say that the number of regions of electron density is 5.
We should know that the middle sulphur atom containing the 5 valence atomic orbitals is basically hybridized to form five \[s{{p}^{3}}d\] hybrid orbitals. So, from this discussion we can say that the hybridisation of sulphur as the central atom in sulphur tetrafluoride is \[s{{p}^{3}}d\].
So, the correct answer to this question is option C.
Note: We should know some important points. We should know that the central sulphur atom has one lone pair and is bonded to four fluorine atoms. And by this statement we can say that there are five hybrid orbitals formed. We should know about these five hybrid orbitals that it has one 3s-orbital, three 3p-orbitals and one 3d-orbital participate in hybridization. And one important thing that we should know is \[S{{F}_{4}}\] molecular geometry is seesaw with one pair of valence electrons.
Complete step by step solution:
To answer the hybridisation of sulphur in sulfur tetrafluoride, we have to first understand its Lewis structure and the number of valence electrons that are present. The \[S{{F}_{4}}\] molecule consists of a total of 34 valence electrons. Here 6 will come from sulfur and each of the four fluorine atoms will have 7 electrons.
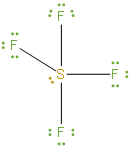
The above structure is the Lewis dot structure of \[S{{F}_{4}}\].
We should note that during the formation of \[S{{F}_{4}}\], the sulphur atom will form bonds with each of fluorine atoms in which 8 of valence electrons are used. And we can say that, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. And also we can say that two electrons will be kept as lone pairs in the sulphur atom. Now we can determine sulphur’s hybridization by taking a count of the number of regions of electron density. When bonding takes place there is a formation of 4 single bonds in sulphur and it has 1 lone pair. Looking at this, we can say that the number of regions of electron density is 5.
We should know that the middle sulphur atom containing the 5 valence atomic orbitals is basically hybridized to form five \[s{{p}^{3}}d\] hybrid orbitals. So, from this discussion we can say that the hybridisation of sulphur as the central atom in sulphur tetrafluoride is \[s{{p}^{3}}d\].
So, the correct answer to this question is option C.
Note: We should know some important points. We should know that the central sulphur atom has one lone pair and is bonded to four fluorine atoms. And by this statement we can say that there are five hybrid orbitals formed. We should know about these five hybrid orbitals that it has one 3s-orbital, three 3p-orbitals and one 3d-orbital participate in hybridization. And one important thing that we should know is \[S{{F}_{4}}\] molecular geometry is seesaw with one pair of valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




