
The hybridisation of carbon in diamond, graphite and acetylene is respectively:
(A).$s{p^3}$, $sp$, $s{p^2}$
(B).sp, $s{p^2}$, $s{p^3}$
(C).$s{p^3}$, $s{p^2}$, sp
(D).$s{p^2}$, $s{p^3}$, sp
Answer
595.2k+ views
Hint: Diamond forms a covalent and cubic structure with small tetrahedron units. Graphite exists in layers with hexagonal rings. The IUPAC name of acetylene is ethyne and the suffix (-yne) shows that it is an unsaturated compound.
Complete answer:
We should start by seeing these 3 compounds individually.
-We should know that graphite and diamond, both are very stable allotropes of carbon. But graphite is the most stable.
-Let’s talk about diamond first. Diamond is an allotrope of carbon which forms a giant covalent and cubic crystal structure by forming a repeating pattern of 8 atoms. All the carbon atoms in diamond form strong chemical bonds with other 4 carbon atoms which makes a perfect tetrahedron structure in the entire diamond structure.
Since each carbon atom is forming 4 strong sigma bonds, one with each of the 4 other carbon atoms, its hybridisation will be $s{p^3}$.
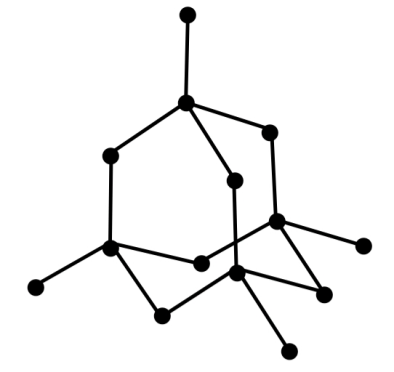
-About graphite we already know that it is the most stable allotrope of carbon. Graphite forms a giant covalent structure where every carbon atom forms 3 strong sigma bonds, one with each of the 3 other carbon atoms. This means that every carbon atom is bonded with 3 other carbon atoms. These carbon atoms form layers of hexagonal rings. Although these layers are attached to each other by weak forces.
Since here every carbon atom is attached to 3 other carbon atoms, the hybridisation of C is $s{p^2}$.
-Now let us see acetylene. Acetylene is an organic compound with a molecular formula of: ${C_2}{H_2}$ and its IUPAC name is ethyne. It is an unsaturated compound. Here the 2 carbon atoms are attached to each other via a triple bond (1 sigma bond and 2 pi bonds). Hence the hybridisation of acetylene will be sp.

-So, the correct option is: (C)$s{p^3}$, $s{p^2}$, sp.
Additional information:
Carbon has the ability to form 8 different types of allotropes. All have different structures, hardness, properties, etc. On one hand where graphite is very soft due to its layered structure, while on the other hand diamond is very difficult to break and is used to cut glass.
Note: Remember acetylene is not the IUPAC name, it is a common name. Sometimes we just see the suffix (–ene) and assume the compound to be having a double bond and so $s{p^2}$ structure. This is false since the IUPAC name is ethyne.
Complete answer:
We should start by seeing these 3 compounds individually.
-We should know that graphite and diamond, both are very stable allotropes of carbon. But graphite is the most stable.
-Let’s talk about diamond first. Diamond is an allotrope of carbon which forms a giant covalent and cubic crystal structure by forming a repeating pattern of 8 atoms. All the carbon atoms in diamond form strong chemical bonds with other 4 carbon atoms which makes a perfect tetrahedron structure in the entire diamond structure.
Since each carbon atom is forming 4 strong sigma bonds, one with each of the 4 other carbon atoms, its hybridisation will be $s{p^3}$.

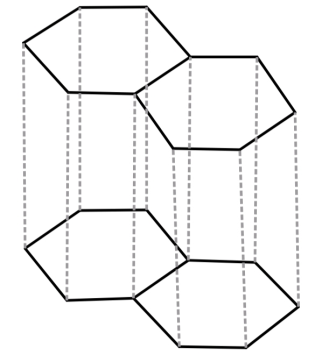
-About graphite we already know that it is the most stable allotrope of carbon. Graphite forms a giant covalent structure where every carbon atom forms 3 strong sigma bonds, one with each of the 3 other carbon atoms. This means that every carbon atom is bonded with 3 other carbon atoms. These carbon atoms form layers of hexagonal rings. Although these layers are attached to each other by weak forces.
Since here every carbon atom is attached to 3 other carbon atoms, the hybridisation of C is $s{p^2}$.

-Now let us see acetylene. Acetylene is an organic compound with a molecular formula of: ${C_2}{H_2}$ and its IUPAC name is ethyne. It is an unsaturated compound. Here the 2 carbon atoms are attached to each other via a triple bond (1 sigma bond and 2 pi bonds). Hence the hybridisation of acetylene will be sp.

-So, the correct option is: (C)$s{p^3}$, $s{p^2}$, sp.
Additional information:
Carbon has the ability to form 8 different types of allotropes. All have different structures, hardness, properties, etc. On one hand where graphite is very soft due to its layered structure, while on the other hand diamond is very difficult to break and is used to cut glass.
Note: Remember acetylene is not the IUPAC name, it is a common name. Sometimes we just see the suffix (–ene) and assume the compound to be having a double bond and so $s{p^2}$ structure. This is false since the IUPAC name is ethyne.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




