
The hybridisation and geometry of $B{{F}_{3}}$ molecule is:
(A) $s{{p}^{3}}d$ and T-shaped
(B) $s{{p}^{3}}{{d}^{2}}$ and tetragonal
(C) $s{{p}^{3}}d$ and bent
(D) none of these
Answer
585.6k+ views
Hint: The Lewis dot structure is used involving the violation of octet rule. Further, to determine the shape, the orbitals in the central metal atom undergoes intermixing of its valence shell orbitals to prevent the repulsion. Thereby, predicting the shape and hybridisation of the molecule.
Complete step by step solution:
With the help of VSEPR theory, the shape/geometry of boron trifluoride can be determined. It takes into account the repulsion between the valence electron pairs in all the atoms, as they orient themselves to attain the geometry which minimises the repulsion in the resulting molecule.
- The boron atom is the central atom which is the least electronegative atom having the highest ability to share its electrons with the neighbouring atoms. Boron with atomic number = 5, has a valence shell configuration as $2{{s}^{2}}2{{p}^{1}}$, that is, three valence electrons.
-In the excited state, the one 2s electron jumps to the 2p orbital, producing three unpaired electrons. This further leads to the formation of three $s{{p}^{2}}$ hybridised orbital, with equal energies and containing one electron each.
-The fluorine atom with the seven valence electrons $(2{{s}^{2}}2{{p}^{5}})$. These half-filled p-orbitals of the three fluorine atoms overlap with $s{{p}^{2}}$ hybridised orbitals of boron atoms, forming three coplanar B-F electron-pair bonds. Thus, having $s{{p}^{2}}$hybridization.
For the Boron atom, the total number of electron pairs around it is,
$=\dfrac{1}{2}\times (\text{no}\text{. of valence electrons + no}\text{.of atoms linked by single bonds)}$
\[=\dfrac{1}{2}(3+3)=3\]
-Then, the number of bond pairs is three which is equal to the number of atoms linked to boron atoms by single bonds.
-It has zero lone pairs as the electron pair is equal to the number of shared pairs.
Thus, we get the VSEP number equal to 3. Then, the possible shape will be trigonal planar to minimise the repulsion.
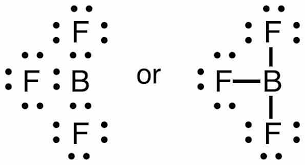
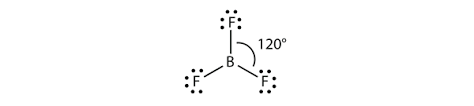
Thus, the boron fluoride molecule with $s{{p}^{2}}$ hybridisation and trigonal planar geometry is an option (D)- None of these.
Note: Here, the boron atom with one empty p-orbital accepts an electron pair from any of the three fluorine atoms. Thus, forming a partial double bond and a resonance hybrid is produced. Though these forms are not readily formed.
Complete step by step solution:
With the help of VSEPR theory, the shape/geometry of boron trifluoride can be determined. It takes into account the repulsion between the valence electron pairs in all the atoms, as they orient themselves to attain the geometry which minimises the repulsion in the resulting molecule.
- The boron atom is the central atom which is the least electronegative atom having the highest ability to share its electrons with the neighbouring atoms. Boron with atomic number = 5, has a valence shell configuration as $2{{s}^{2}}2{{p}^{1}}$, that is, three valence electrons.
-In the excited state, the one 2s electron jumps to the 2p orbital, producing three unpaired electrons. This further leads to the formation of three $s{{p}^{2}}$ hybridised orbital, with equal energies and containing one electron each.
-The fluorine atom with the seven valence electrons $(2{{s}^{2}}2{{p}^{5}})$. These half-filled p-orbitals of the three fluorine atoms overlap with $s{{p}^{2}}$ hybridised orbitals of boron atoms, forming three coplanar B-F electron-pair bonds. Thus, having $s{{p}^{2}}$hybridization.
For the Boron atom, the total number of electron pairs around it is,
$=\dfrac{1}{2}\times (\text{no}\text{. of valence electrons + no}\text{.of atoms linked by single bonds)}$
\[=\dfrac{1}{2}(3+3)=3\]
-Then, the number of bond pairs is three which is equal to the number of atoms linked to boron atoms by single bonds.
-It has zero lone pairs as the electron pair is equal to the number of shared pairs.
Thus, we get the VSEP number equal to 3. Then, the possible shape will be trigonal planar to minimise the repulsion.


Thus, the boron fluoride molecule with $s{{p}^{2}}$ hybridisation and trigonal planar geometry is an option (D)- None of these.
Note: Here, the boron atom with one empty p-orbital accepts an electron pair from any of the three fluorine atoms. Thus, forming a partial double bond and a resonance hybrid is produced. Though these forms are not readily formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




