
The Hofmann rearrangement of an amide, $RCON{H_2}$ to an amine $RN{H_2}$, is brought about by treating the amide with bromine in aqueous sodium hydride. Which of the species below is not an intermediate in Hofmann rearrangement?
A.$RNHCOOH$
B.$RN = C = O$
C.$RCONHR$
D.$RCON{H^ - }$
Answer
590.4k+ views
Hint:When a primary amide is treated with an aqueous or ethanolic solution of potassium hydroxide and bromine or potassium hypobromite (\[KOBr\]) it gives primary amine which has one carbon less than the original amide. This reaction is also known as the Hofmann bromamide reaction.
Complete step by step answer:Hofmann bromide or Hofmann degradation of primary amides reaction is given as:
$RCON{H_2} + B{r_2} + 4KOH \to RN{H_2} + {K_2}C{O_3} + 2KBr + 2{H_2}O$
This method is widely used for stepping down or descent homologous series. The mechanism of Hofmann's bromide reaction involves the formation of acyl nitrene as an intermediate which undergoes rearrangement to give alkyl isocyanate as an intermediate. The mechanism of Hofmann degradation reaction is given as:
Step1: This step involves the attack of a strong base to an amide.
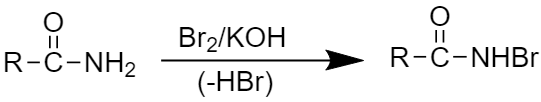
Step2: This step involves the formation of intermediate acyl nitrene.
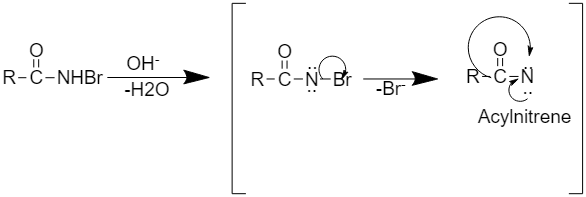
Step 3: This step involves the rearrangement to form alkyl isocyanate
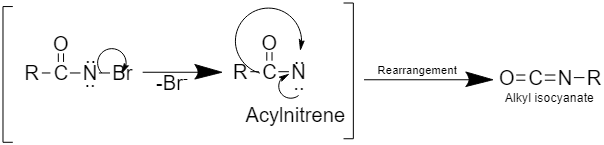
Step 4: This step involves the formation of primary amine by hydrolysis of alkyl isocyanate as:
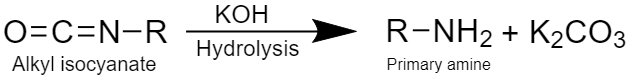
During the mechanism of the reaction, the intermediate compound formed is alkyl isocyanate
($RN = C = O$).
This mechanism has been supported by the observation that N-bromoamide and alkyl isocyanates have been isolated as intermediates in the above reaction.
Hence the correct answer is option B.
Additional information: In this reaction, the only primary amide is used for the formation of primary amine. We cannot use secondary and tertiary amide for the preparation of primary amine in this reaction.
Note: The formation of isocyanate as an intermediate in the above reaction is supported by the observation that when this reaction is carried out with methanolic $C{H_3}ONa$ instead of aqueous $NaOH$, a methyl carbamate or urethane is obtained instead of the amine.
Complete step by step answer:Hofmann bromide or Hofmann degradation of primary amides reaction is given as:
$RCON{H_2} + B{r_2} + 4KOH \to RN{H_2} + {K_2}C{O_3} + 2KBr + 2{H_2}O$
This method is widely used for stepping down or descent homologous series. The mechanism of Hofmann's bromide reaction involves the formation of acyl nitrene as an intermediate which undergoes rearrangement to give alkyl isocyanate as an intermediate. The mechanism of Hofmann degradation reaction is given as:
Step1: This step involves the attack of a strong base to an amide.

Step2: This step involves the formation of intermediate acyl nitrene.

Step 3: This step involves the rearrangement to form alkyl isocyanate

Step 4: This step involves the formation of primary amine by hydrolysis of alkyl isocyanate as:

During the mechanism of the reaction, the intermediate compound formed is alkyl isocyanate
($RN = C = O$).
This mechanism has been supported by the observation that N-bromoamide and alkyl isocyanates have been isolated as intermediates in the above reaction.
Hence the correct answer is option B.
Additional information: In this reaction, the only primary amide is used for the formation of primary amine. We cannot use secondary and tertiary amide for the preparation of primary amine in this reaction.
Note: The formation of isocyanate as an intermediate in the above reaction is supported by the observation that when this reaction is carried out with methanolic $C{H_3}ONa$ instead of aqueous $NaOH$, a methyl carbamate or urethane is obtained instead of the amine.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




