
The heat of hydrogenation of cyclohexene is $28.6\;Kcal$ and that of cyclohexadiene is about twice as much of cyclohexene$\left( {i.e.,\;55.4Kcal} \right)$ . Then what would be the heat of hydrogenation of benzene, which has three double bonds?
A. Thrice that of cyclohexene $\left( {28.63Kcal} \right)$
B. The same as that of cyclohexene
C. The same as that of cyclohexadiene
D. $49.8Kcal$
Answer
563.4k+ views
Hint:We are going to write down the heat of hydrogenation$\left( {\vartriangle {H_{hyd}}} \right)$ of each compound given along with their structure for better understanding. The compound with single double bond is cyclohexene , cyclohexadiene has two double bonds and benzene has three double bonds.
Complete step-by-step solution:In order to solve this question we are going to draw the structure of cyclohexene ,cyclohexadiene and benzene.
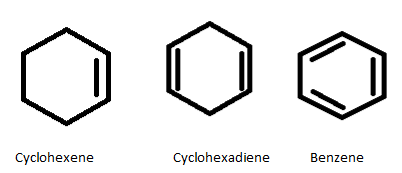
In this question we have been given the heat of hydrogenation$\left( {i.e.,\;55.4Kcal} \right)$ of cyclohexene is $28.6\;Kcal$ and heat of hydrogenation of cyclohexadiene is twice of cyclohexene $\left( {i.e.,\;55.4Kcal} \right)$ .
We are asked to find the heat of hydrogenation$\left( {i.e.,\;55.4Kcal} \right)$ of benzene ,which has three double bonds.
So to proceed further, we know that,
${C_6}{H_{10}} + {C_6}{H_8} \to {C_6}{H_6}$
Here cyclohexene has one double bond so, counting the number of carbon and hydrogen , the molecular formula for cyclohexene is ${C_6}{H_{10}}$
Cyclohexadiene has two double bonds , counting the number of carbon and hydrogen , the molecular formula for cyclohexane is ${C_6}{H_8}$
Benzene has three double bonds, counting the number of carbon and hydrogen , the molecular formula for Benzene is ${C_6}{H_6}$
So adding up the amount of heat of hydrogenation we get,
$28.6 + 55.4$
Which gives $ = 84$
Which is approximately thrice of $28.6$ i.e., Thrice of cyclohexene.
So among all the options, option A. is correct.
Note:Students can make mistakes in writing the correct structure of the compounds . Knowing the correct molecular formula and forming the equation of the compounds is important . Also an important concept here to keep in mind is the concept of heat of hydrogenation.
Complete step-by-step solution:In order to solve this question we are going to draw the structure of cyclohexene ,cyclohexadiene and benzene.

In this question we have been given the heat of hydrogenation$\left( {i.e.,\;55.4Kcal} \right)$ of cyclohexene is $28.6\;Kcal$ and heat of hydrogenation of cyclohexadiene is twice of cyclohexene $\left( {i.e.,\;55.4Kcal} \right)$ .
We are asked to find the heat of hydrogenation$\left( {i.e.,\;55.4Kcal} \right)$ of benzene ,which has three double bonds.
So to proceed further, we know that,
${C_6}{H_{10}} + {C_6}{H_8} \to {C_6}{H_6}$
Here cyclohexene has one double bond so, counting the number of carbon and hydrogen , the molecular formula for cyclohexene is ${C_6}{H_{10}}$
Cyclohexadiene has two double bonds , counting the number of carbon and hydrogen , the molecular formula for cyclohexane is ${C_6}{H_8}$
Benzene has three double bonds, counting the number of carbon and hydrogen , the molecular formula for Benzene is ${C_6}{H_6}$
So adding up the amount of heat of hydrogenation we get,
$28.6 + 55.4$
Which gives $ = 84$
Which is approximately thrice of $28.6$ i.e., Thrice of cyclohexene.
So among all the options, option A. is correct.
Note:Students can make mistakes in writing the correct structure of the compounds . Knowing the correct molecular formula and forming the equation of the compounds is important . Also an important concept here to keep in mind is the concept of heat of hydrogenation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




