
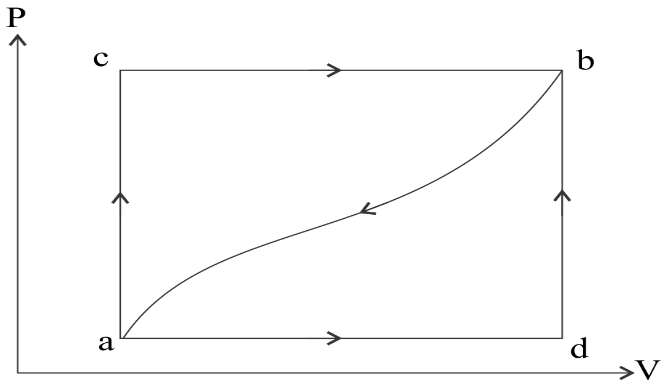
What will be the heat absorbed if the work done on the system along the curved path ‘ba’ is 52 J.
A.-140 J
B.-172 J
C.140 J
D.172 J

Answer
576k+ views
Hint: Every point on a PV diagram represents a different state for the gas (one for every possible volume and pressure). As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves. Being able to decode the information shown in a PV diagram allows us to make statements about the change in internal energy $\Delta U$, heat transferred Q, and work done W on a gas.
Complete answer:
-If we press the piston downwards, the volume of the gas will decrease, so the state must shift to the left toward smaller volumes. Since the gas is being compressed we can also say for sure that positive work W is being done on the gas.
-Similarly, if we let the gas expand, pushing the piston upward, the volume of the gas will increase, so the state must shift to the right toward larger volumes. Since the gas is expanding we can also say for sure that negative work W is being done on the gas.
-The work done during a thermodynamic process is equal to the area under the curve.
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase.
-We know that,
$\Delta U = Q - W$ here,$\Delta U=-120$ and $W = - 52J$
Substituting the values
$
Q = \Delta U - W \\
Q = - 120 - 52 \\
Q = - 172J \\
$
Hence, the correct answer is (B).
Note:
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase. So if the quantity of pressure times volume increases, the temperature T and internal energy U must also increase.
Complete answer:
-If we press the piston downwards, the volume of the gas will decrease, so the state must shift to the left toward smaller volumes. Since the gas is being compressed we can also say for sure that positive work W is being done on the gas.
-Similarly, if we let the gas expand, pushing the piston upward, the volume of the gas will increase, so the state must shift to the right toward larger volumes. Since the gas is expanding we can also say for sure that negative work W is being done on the gas.
-The work done during a thermodynamic process is equal to the area under the curve.
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase.
-We know that,
$\Delta U = Q - W$ here,$\Delta U=-120$ and $W = - 52J$
Substituting the values
$
Q = \Delta U - W \\
Q = - 120 - 52 \\
Q = - 172J \\
$
Hence, the correct answer is (B).
Note:
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase. So if the quantity of pressure times volume increases, the temperature T and internal energy U must also increase.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




