
The graph for Boyle’s law is called:
A.Isotherm
B.Hypertherm
C.Hypertherm
D.None of the above
Answer
583.8k+ views
Hint: Basically, Boyle’s law is a gas law which states that the pressure exerted by a gas is inversely proportional to the volume occupied by it. The graph of this law is generally plotted between V vs P at constant temperature for a fixed mass of a gas.
Complete step by step answer:
Boyle’s law states that the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quality of gas are kept constant. This law was given by Anglo Irish chemist Robert Boyle in the year 1662.
Now, the relationship between the volume and pressure can be expressed as:
$P\alpha \dfrac{1}{V}$
Where P is the pressure exerted by the gas and V is the volume occupied.
Further, this relation can be converted into an equation by adding a constant ‘k’. The equation is as shown:
$P = k(\dfrac{1}{V})$
$PV = k$
Further, this law can also be expressed as:
${P_1}{V_1} = {P_2}{V_2}$
Where ${P_1}$ and ${P_2}$ are initial and final pressures exerted by the gas whereas ${V_1}$ and ${V_2}$ are initial and final volumes occupied by the gas.
Now, the pressure $\dfrac{v}{s}$ volume curve for a fixed amount of gas at constant temperature is as shown:
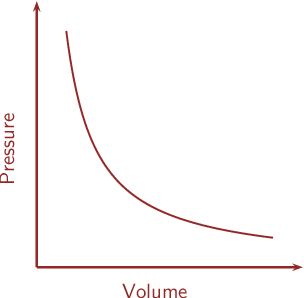
Now, the graph of Boyle’s law is called an isotherm because iso means the same and term means thermal or temperature and hence the temperature remains constant.
Hence, option A is correct.
Note: Some of the applications of Boyle’s law in our daily life are soda can, spray paint, drawing fluid into a syringe and many more. Moreover, if a scuba diver rapidly ascends from a deep zone towards the surface of water, the decrease in pressure can cause the gas molecules in the body to expand and these gas bubbles can cause damage to the diver's organs and may also cause death. This expansion of gas is also an example of Boyle’s law.
Complete step by step answer:
Boyle’s law states that the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quality of gas are kept constant. This law was given by Anglo Irish chemist Robert Boyle in the year 1662.
Now, the relationship between the volume and pressure can be expressed as:
$P\alpha \dfrac{1}{V}$
Where P is the pressure exerted by the gas and V is the volume occupied.
Further, this relation can be converted into an equation by adding a constant ‘k’. The equation is as shown:
$P = k(\dfrac{1}{V})$
$PV = k$
Further, this law can also be expressed as:
${P_1}{V_1} = {P_2}{V_2}$
Where ${P_1}$ and ${P_2}$ are initial and final pressures exerted by the gas whereas ${V_1}$ and ${V_2}$ are initial and final volumes occupied by the gas.
Now, the pressure $\dfrac{v}{s}$ volume curve for a fixed amount of gas at constant temperature is as shown:

Now, the graph of Boyle’s law is called an isotherm because iso means the same and term means thermal or temperature and hence the temperature remains constant.
Hence, option A is correct.
Note: Some of the applications of Boyle’s law in our daily life are soda can, spray paint, drawing fluid into a syringe and many more. Moreover, if a scuba diver rapidly ascends from a deep zone towards the surface of water, the decrease in pressure can cause the gas molecules in the body to expand and these gas bubbles can cause damage to the diver's organs and may also cause death. This expansion of gas is also an example of Boyle’s law.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




