
The given table shows the properties of four cell systems A, B, C, and D. The maximum rate of inward diffusion of water will be observed in which of these systems?
System The intracellular concentration of water Extracellular concentration of water A 0.09M 0.11M B 0.2M 0.5M C 0.05M 0.7M D 0.03M 0.6M
(a) System A
(b) System B
(c) System C
(d) System D
| System | The intracellular concentration of water | Extracellular concentration of water |
| A | 0.09M | 0.11M |
| B | 0.2M | 0.5M |
| C | 0.05M | 0.7M |
| D | 0.03M | 0.6M |
Answer
586.5k+ views
Hint: Diffusion of water across a semipermeable membrane is particularly referred to as osmosis. Movement of water molecules occurs from their higher concentration to their lower concentration until the concentration of both the solution becomes equal.
Complete step by step answer:
Osmotic pressure is the actual pressure that develops in a solution when it is separated from water by means of a semi- permeable membrane or it is the pressure needed to prevent the passage of water into the solution through the semipermeable membrane.
The more the concentration of solutes in a solution, the more pressure is required to prevent the influx of water inside and thus, the more osmotic pressure. In the given set of systems, system C has the highest difference in the concentration between the sets of solutions and thus the highest osmotic pressure due to the maximum rate of inward diffusion of water.
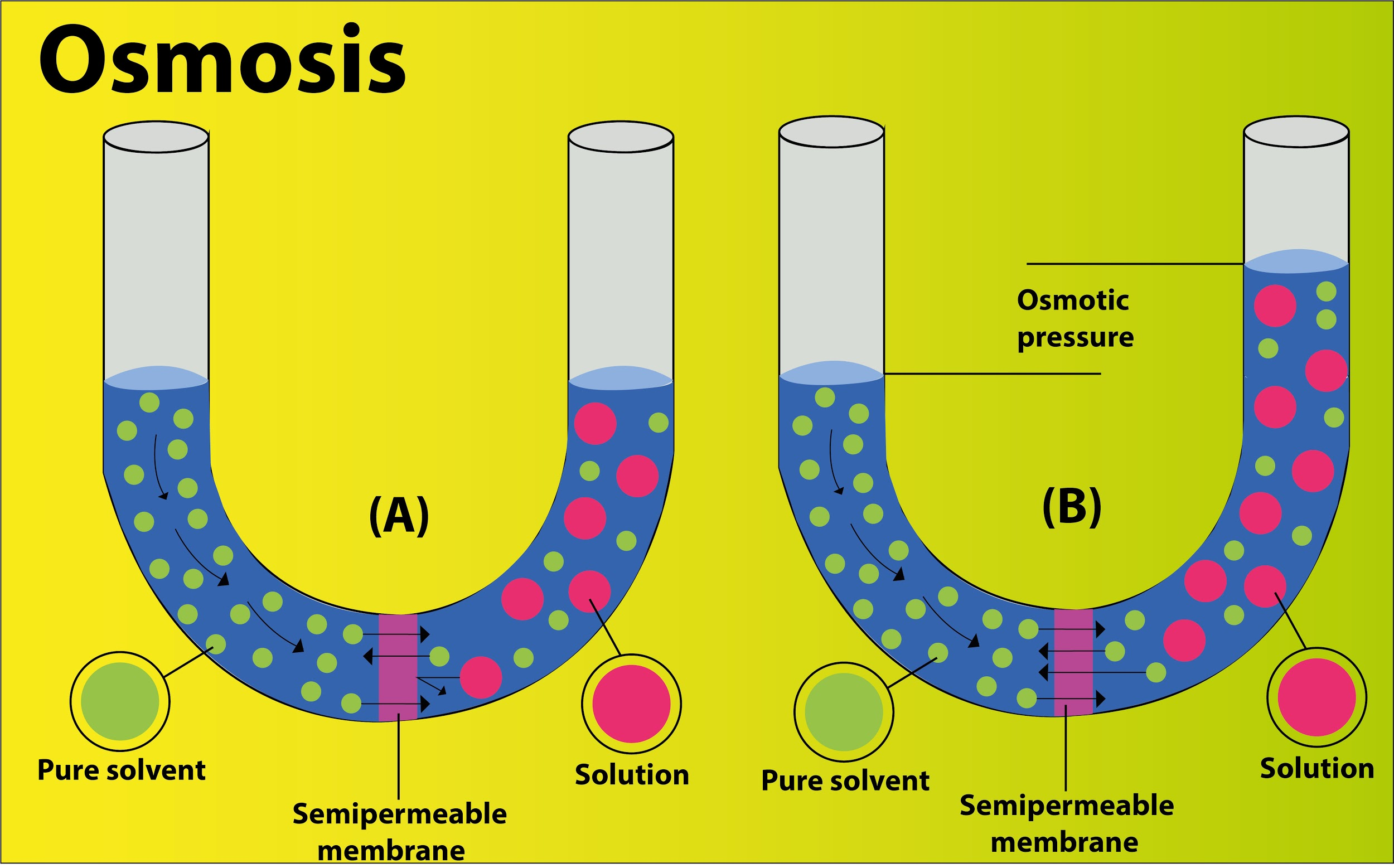
- Three types of solutions can be observed in the living world based on the osmotic potential- Isotonic solution, Hypotonic solution, and Hypertonic solution.
- When the concentration of the outer solution in which a cell is placed is equal to the concentration of cell sap, it is called an isotonic solution.
- When the concentration of the outer solution is higher than that of the cell sap, then such a system is referred to as a hypertonic solution.
- If the concentration of the outer solution is lower than the concentration of the cell sap, then the solution is known as a hypotonic solution.
So, the correct answer is ‘(c) System C.’
Note:
- No net movement of water across the semipermeable membrane occurs if a cell is placed in an isotonic solution and thus no change in size can be observed.
- When a cell is placed in a hypertonic solution such as salt water, the water from the cell tends to move outside. It will lead to shrinkage in cell size.
- A cell placed in a hypotonic solution will swell due to the influx of water into the cell sap.
Complete step by step answer:
Osmotic pressure is the actual pressure that develops in a solution when it is separated from water by means of a semi- permeable membrane or it is the pressure needed to prevent the passage of water into the solution through the semipermeable membrane.
The more the concentration of solutes in a solution, the more pressure is required to prevent the influx of water inside and thus, the more osmotic pressure. In the given set of systems, system C has the highest difference in the concentration between the sets of solutions and thus the highest osmotic pressure due to the maximum rate of inward diffusion of water.

- Three types of solutions can be observed in the living world based on the osmotic potential- Isotonic solution, Hypotonic solution, and Hypertonic solution.
- When the concentration of the outer solution in which a cell is placed is equal to the concentration of cell sap, it is called an isotonic solution.
- When the concentration of the outer solution is higher than that of the cell sap, then such a system is referred to as a hypertonic solution.
- If the concentration of the outer solution is lower than the concentration of the cell sap, then the solution is known as a hypotonic solution.
So, the correct answer is ‘(c) System C.’
Note:
- No net movement of water across the semipermeable membrane occurs if a cell is placed in an isotonic solution and thus no change in size can be observed.
- When a cell is placed in a hypertonic solution such as salt water, the water from the cell tends to move outside. It will lead to shrinkage in cell size.
- A cell placed in a hypotonic solution will swell due to the influx of water into the cell sap.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




