
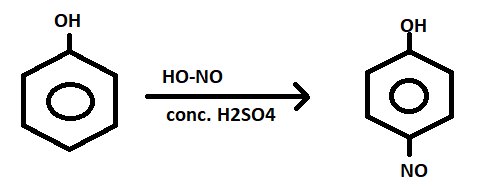
The given reaction is called:
$Phenol{\text{ }}\frac{{conc.{H_2}S{O_4}}}{{NaN{O_2}}} > p - nitrosophenol$
$
{\text{A}}{\text{. Liebermann's nitroso reaction}} \\
{\text{B}}{\text{. Lederer - manasse reaction}} \\
{\text{C}}{\text{. Fries rearrangement}} \\
{\text{D}}{\text{. Reimer - Tiemann reaction}} \\
$
Answer
609k+ views
Hint: In this reaction, the product gives a violet-blue color and this is an identification test for phenol group. This is the first step of a name reaction.
Complete answer:
Here, $HN{O_2}$ breaks as HO-NO so here, $N{O^ + }$ acts as electro-phile and attaches at the para-position because phenol has high electron density at its para-position.
On adding further reagents, the reaction proceeds with an attack by the more nucleophilic N.
In absence of sulphuric acid, that is, in a dilute medium, the color changes occur as shown:
In the presence of an acid the color changes occur as:

First, brown red color appears on warming phenol and conc. $H_2SO_4$.
This red then changes to blue then green.
On dilution, color changes to red and finally, to greenish blue or violet on treatment with alkali.
The test is given by only 2 degree amines (both aliphatic and aromatic). Two degree amine is converted into nitroso-amine by treating the amine with $HNO_2$. So, this is a detection test for secondary amines. Since, phenol is the starting substrate in this reaction, so this test is an indicator of phenol group as well.
So, the given reaction in the question is the first step of Libermann’s Nitroso reaction.
So the correct option is (A).
Note: Name reactions are really important in chemistry. The particular reagent of the reaction, substrate and the product all should be known correctly to do such questions.
Complete answer:
Here, $HN{O_2}$ breaks as HO-NO so here, $N{O^ + }$ acts as electro-phile and attaches at the para-position because phenol has high electron density at its para-position.
On adding further reagents, the reaction proceeds with an attack by the more nucleophilic N.

In absence of sulphuric acid, that is, in a dilute medium, the color changes occur as shown:
In the presence of an acid the color changes occur as:

First, brown red color appears on warming phenol and conc. $H_2SO_4$.
This red then changes to blue then green.
On dilution, color changes to red and finally, to greenish blue or violet on treatment with alkali.
The test is given by only 2 degree amines (both aliphatic and aromatic). Two degree amine is converted into nitroso-amine by treating the amine with $HNO_2$. So, this is a detection test for secondary amines. Since, phenol is the starting substrate in this reaction, so this test is an indicator of phenol group as well.
So, the given reaction in the question is the first step of Libermann’s Nitroso reaction.
So the correct option is (A).
Note: Name reactions are really important in chemistry. The particular reagent of the reaction, substrate and the product all should be known correctly to do such questions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




