
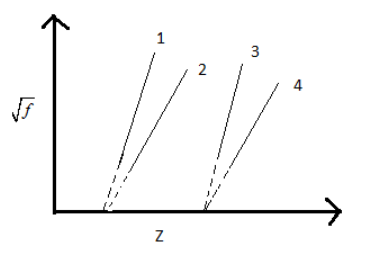
The given graph shows the variation of $\sqrt{f}$ vs Z for characteristics X-rays. Lines 1, 2, 3, 4 shown in the graph corresponds to any one of ${{K}_{\alpha }}$, ${{K}_{\beta }}$, ${{L}_{\alpha }}$ and ${{L}_{\beta }}$, then line ${{L}_{\beta }}$ is represented by (f=frequency, Z =atomic number)
(A)Line 1
(B)Line 2
(C)Line 3
(D)Line 4

Answer
582.9k+ views
Hint: The four lines represent the 4 lines of characteristic X ray given as ${{K}_{\alpha }}$, ${{K}_{\beta }}$, ${{L}_{\alpha }}$ and ${{L}_{\beta }}$. To deduce which line represents which we must understand the energy values of these lines and try to establish a relationship among them.
Complete answer:
We know,
In characteristic X rays, the K series of lines have higher energy values than the L series of lines ....................... (1)
Moreover, we have
The $\beta $ lines are more energetic in comparison to the $\alpha $ lines. .................. (2)
We know,
Energy is directly proportional to the frequency. So more is the energy, more is the frequency which is represented by the y-axis. ................... (3)
Using the information given by (1), (2) and (3), we can arrange the lines in ascending or descending order as per our choice and identify the ${{L}_{\beta }}$ line.
In ascending order of frequency, the lines can be arranged as
${{L}_{\alpha }}<{{L}_{\beta }}<{{K}_{\alpha }}<{{K}_{\beta }}$
From the series in ascending order, we can deduce that the ${{L}_{\beta }}$line is the 3rd line.
So the correct answer is (C) Line 3.
Additional Information:
The study of atomic spectra which is based on some set of Selection Rules is a very important source which provides us the knowledge of atoms and molecules. Characteristic X rays are emitted when the outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern which is the characteristic of the element.
Note:
In this problem we must know certain facts. We must understand that the K set of lines are more energetic than the L set of lines and also that the $\beta $ lines are more energetic than the $\alpha $ . Also we must understand the relationship between energy and frequency and relate the three sets of information into finding the ${{L}_{\beta }}$ line.
Complete answer:
We know,
In characteristic X rays, the K series of lines have higher energy values than the L series of lines ....................... (1)
Moreover, we have
The $\beta $ lines are more energetic in comparison to the $\alpha $ lines. .................. (2)
We know,
Energy is directly proportional to the frequency. So more is the energy, more is the frequency which is represented by the y-axis. ................... (3)
Using the information given by (1), (2) and (3), we can arrange the lines in ascending or descending order as per our choice and identify the ${{L}_{\beta }}$ line.
In ascending order of frequency, the lines can be arranged as
${{L}_{\alpha }}<{{L}_{\beta }}<{{K}_{\alpha }}<{{K}_{\beta }}$
From the series in ascending order, we can deduce that the ${{L}_{\beta }}$line is the 3rd line.
So the correct answer is (C) Line 3.
Additional Information:
The study of atomic spectra which is based on some set of Selection Rules is a very important source which provides us the knowledge of atoms and molecules. Characteristic X rays are emitted when the outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern which is the characteristic of the element.
Note:
In this problem we must know certain facts. We must understand that the K set of lines are more energetic than the L set of lines and also that the $\beta $ lines are more energetic than the $\alpha $ . Also we must understand the relationship between energy and frequency and relate the three sets of information into finding the ${{L}_{\beta }}$ line.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




