
The given compound is a projection formula of:
(a) Cyclohexane
(b) Cyclopentane
(c) Cyclobutane
(d) Cyclopropane
Answer
541.8k+ views
Hint :As we know that Cyclic compounds are the organic compounds which are also known as ring structure. These structures can contain various carbon atoms with single, double and triple bonds. The cyclic compounds are saturated compounds. The bridged cycloalkanes contain one or more pairs of carbon atoms which are common to two or more rings.
Complete Step By Step Answer:
We know that the given structure can be modified into the following structure:
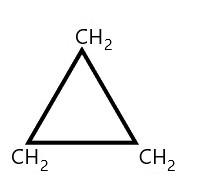
On seeing the above structure, we can see that three carbon atoms are attached to the ring and they are bonded by a single bond so the structure formed is called cyclopropane. So, the correct answer for this question is option ‘d’.
The different types of cyclic forms of ring are:

The above structure is known as cyclohexene.
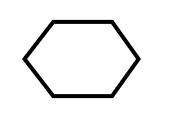
The above structure is known as cyclohexane.
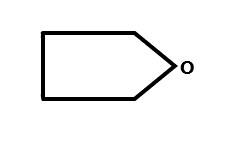
The above structure is known as tetrahydrofuran.
In aromatic hydrocarbons the ring structure contains six carbon atoms which is generally known as benzene ring. The bonds in this benzene ring are arranged alternatively with single and double bonds. In cyclic hydrocarbons if we omit hydrogen on any of the carbon atoms then it is called aryl hydrocarbon.
Note :
Remember that the aryl hydrocarbons are reactive in nature and get bound with a functional group. The cycloalkane are saturated hydrocarbons and have high melting and boiling point. They are insoluble in water and they are said to be solvent in liquid form. If the molecule gets burned then it will get destroyed.
Complete Step By Step Answer:
We know that the given structure can be modified into the following structure:

On seeing the above structure, we can see that three carbon atoms are attached to the ring and they are bonded by a single bond so the structure formed is called cyclopropane. So, the correct answer for this question is option ‘d’.
The different types of cyclic forms of ring are:

The above structure is known as cyclohexene.

The above structure is known as cyclohexane.

The above structure is known as tetrahydrofuran.
In aromatic hydrocarbons the ring structure contains six carbon atoms which is generally known as benzene ring. The bonds in this benzene ring are arranged alternatively with single and double bonds. In cyclic hydrocarbons if we omit hydrogen on any of the carbon atoms then it is called aryl hydrocarbon.
Note :
Remember that the aryl hydrocarbons are reactive in nature and get bound with a functional group. The cycloalkane are saturated hydrocarbons and have high melting and boiling point. They are insoluble in water and they are said to be solvent in liquid form. If the molecule gets burned then it will get destroyed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




