
The geometry of $Ni{{\left( CO \right)}_{4}}$ is:
A. Tetrahedral
B. Octahedral
C. Square planar
D. Pyramidal
Answer
577.2k+ views
Hint: The geometry of a complex is defined by the nature of the complex it has which certainly has an effect on the hybridization of the complex.
$CO$ is a strong field ligand in the complex a strong field ligand induces back electron pairing from the valence shell of nickel.
Change in electronic configuration of nickel takes place when $CO$ ligand approaches it.
Complete step by step answer:
The stable state electronic configuration of Nickel is
Argon$\left[ 3{{d}^{8}}4{{s}^{2}}4{{p}^{0}} \right]$.
The valency of Nickel is zero because in the complex, $CO$ is a neutral ligand.
Hence, no electrons will be removed from the above stable state configuration of nickel.
$CO$ is also a strong field ligand and when it approaches nickel, it induces back pairing of electrons from $4s$ to the $3d$ orbital emptying $4s$ orbital.
The new configuration of nickel becomes
argon $\left[ 3{{d}^{10}}4{{s}^{0}}4{{p}^{0}} \right]$
And hence the $3d$ orbital gets filled to 10 electrons making $4s$ orbital and three $4p$orbitals ready for hybridization which decides the geometry and other important properties of the coordinate complex.
All the empty orbitals one $4s$ and three $4p$ mix and hybridize to give $s{{p}^{3}}$ hybridization of the complex.
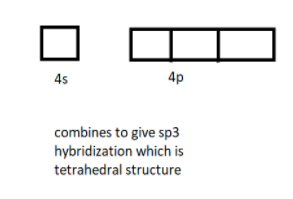
And as we know this type of hybridization corresponds to the tetrahedral structure of the complex. This is a low spin complex.
Hence the correct option for this question is A.
Note: A square planar complex is generally found to form from a strong ligand and a tetrahedral complex is generally found to be formed from weak field ligand.
The procedure used to solve the above question is done in accordance with valence bond theory.
$CO$ is a strong field ligand in the complex a strong field ligand induces back electron pairing from the valence shell of nickel.
Change in electronic configuration of nickel takes place when $CO$ ligand approaches it.
Complete step by step answer:
The stable state electronic configuration of Nickel is
Argon$\left[ 3{{d}^{8}}4{{s}^{2}}4{{p}^{0}} \right]$.
The valency of Nickel is zero because in the complex, $CO$ is a neutral ligand.
Hence, no electrons will be removed from the above stable state configuration of nickel.
$CO$ is also a strong field ligand and when it approaches nickel, it induces back pairing of electrons from $4s$ to the $3d$ orbital emptying $4s$ orbital.
The new configuration of nickel becomes
argon $\left[ 3{{d}^{10}}4{{s}^{0}}4{{p}^{0}} \right]$
And hence the $3d$ orbital gets filled to 10 electrons making $4s$ orbital and three $4p$orbitals ready for hybridization which decides the geometry and other important properties of the coordinate complex.
All the empty orbitals one $4s$ and three $4p$ mix and hybridize to give $s{{p}^{3}}$ hybridization of the complex.

And as we know this type of hybridization corresponds to the tetrahedral structure of the complex. This is a low spin complex.
Hence the correct option for this question is A.
Note: A square planar complex is generally found to form from a strong ligand and a tetrahedral complex is generally found to be formed from weak field ligand.
The procedure used to solve the above question is done in accordance with valence bond theory.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




