
The geometry of $\text{I}_{\text{3}}^{\text{-}}$ is:
(A) Triangular
(B) Linear
(C) Tetrahedral
(D) T-shaped
Answer
560.7k+ views
Hint: The geometry of a molecule is determined according to the hybridisation of the central atom. Geometry of a molecule represents the arrangement of lone pair and bond pair surrounding the central atom.
In $\text{I}_{\text{3}}^{\text{-}}$ ion, central iodine atom have $\text{s}{{\text{p}}^{\text{3}}}$ hybridisation in which one $\text{s}$ -orbital, three $\text{p}$-orbitals and one $\text{d-}$ orbitals are mixed to give five new hybrid orbitals. These orbitals are equivalent in shape and energy called as $\text{s}{{\text{p}}^{\text{3}}}$ hybridised orbitals. The central atom has three lone pairs and two sigma bonds.
Complete step by step answer:
- The geometry of $\text{I}_{\text{3}}^{\text{-}}$ is leaner.
- $\text{I}_{\text{3}}^{\text{-}}$ Ion is made up of a ${{\text{I}}_{2}}$ molecule with a ${{\text{I}}^{-}}$ bonded to it by mean of a coordinate bond in which ${{\text{I}}_{2}}$ molecule is a lone pair acceptor or Lewis acid and ${{\text{I}}^{-}}$ is the lone pair donor or Lewis base.
- There are two bond pairs and three lone pairs in the outer shell of the central atom. Out of five $\text{s}{{\text{p}}^{\text{3}}}\text{d}$ hybrid orbitals of central atom, three are completely filled by lone pair and two are half filled which gets overlaps with two half-filled orbitals of two other iodine atoms.
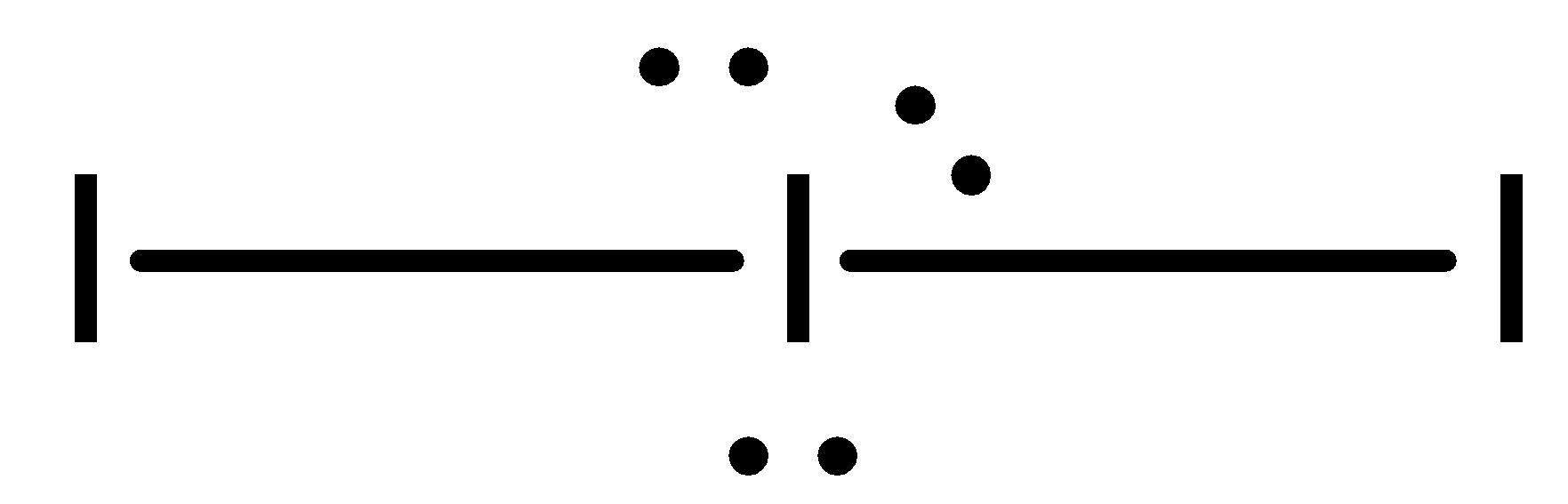
- To minimise the repulsive forces the three lone pairs occupy the equatorial position. The ion is therefore linear in shape with a bond angle of exactly ${{180}^{\circ }}$.
The correct answer is option “B” .
Note: If in a molecule central atom is $\text{s}{{\text{p}}^{\text{3}}}$ hybridised, and have four sigma bond pair and no lone pair, the geometry of the molecule will be tetrahedral.
- If a $\text{s}{{\text{p}}^{\text{3}}}\text{d}$ hybridized molecule have three bond pair and two lone pair, the geometry of the compound will be ‘T’ shape.
In $\text{I}_{\text{3}}^{\text{-}}$ ion, central iodine atom have $\text{s}{{\text{p}}^{\text{3}}}$ hybridisation in which one $\text{s}$ -orbital, three $\text{p}$-orbitals and one $\text{d-}$ orbitals are mixed to give five new hybrid orbitals. These orbitals are equivalent in shape and energy called as $\text{s}{{\text{p}}^{\text{3}}}$ hybridised orbitals. The central atom has three lone pairs and two sigma bonds.
Complete step by step answer:
- The geometry of $\text{I}_{\text{3}}^{\text{-}}$ is leaner.
- $\text{I}_{\text{3}}^{\text{-}}$ Ion is made up of a ${{\text{I}}_{2}}$ molecule with a ${{\text{I}}^{-}}$ bonded to it by mean of a coordinate bond in which ${{\text{I}}_{2}}$ molecule is a lone pair acceptor or Lewis acid and ${{\text{I}}^{-}}$ is the lone pair donor or Lewis base.
- There are two bond pairs and three lone pairs in the outer shell of the central atom. Out of five $\text{s}{{\text{p}}^{\text{3}}}\text{d}$ hybrid orbitals of central atom, three are completely filled by lone pair and two are half filled which gets overlaps with two half-filled orbitals of two other iodine atoms.
- To minimise the repulsive forces the three lone pairs occupy the equatorial position. The ion is therefore linear in shape with a bond angle of exactly ${{180}^{\circ }}$.

The correct answer is option “B” .
Note: If in a molecule central atom is $\text{s}{{\text{p}}^{\text{3}}}$ hybridised, and have four sigma bond pair and no lone pair, the geometry of the molecule will be tetrahedral.
- If a $\text{s}{{\text{p}}^{\text{3}}}\text{d}$ hybridized molecule have three bond pair and two lone pair, the geometry of the compound will be ‘T’ shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




