
The gas liberated by heating potassium permanganate (\[KMn{{O}_{4}}\]) is?
(A) Hydrogen
(B) Oxygen
(C) Nitrogen
(D) Methane
Answer
587.7k+ views
Hint: Whenever we are heating any chemical then the chemical converts from one form to other forms by liberating some gas. Potassium permanganate (\[KMn{{O}_{4}}\]) is a chemical that exists in the solid-state at room temperature.
Complete step by step solution:
-The structure of the potassium permanganate is as follows.
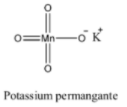
-The reaction of heating of potassium permanganate is as follows.
\[KMn{{O}_{4}}\xrightarrow{\Delta }{{K}_{2}}Mn{{O}_{4}}+Mn{{O}_{2}}+{{O}_{2}}\]
-On heating, potassium permanganate gives potassium manganate, Manganese dioxide and oxygen as the products.
-Coming to given options, option A Hydrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no hydrogen. So, on heating potassium permanganate, hydrogen gas is not going to form as one of the product. Hence option A is wrong.
-Coming option B, Oxygen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is a presence of oxygen. So, on heating potassium permanganate, oxygen gas is going to form as one of the product. Hence option B is correct.
-Coming to option C, Nitrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no nitrogen. So, on heating potassium permanganate, Nitrogen gas is not going to form as one of the product. Hence option C is wrong.
-Coming to option D, Methane. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]) there is no Carbon and hydrogen. So, on heating potassium permanganate, Methane gas is not going to form as one of the product. Hence option D is wrong.
-Therefore on heating potassium permanganate, oxygen gas is going to form as one of the product.
So, the correct option is (B).
Note: The oxidation state of Manganese in potassium permanganate (\[KMn{{O}_{4}}\]) is +7. On heating potassium permanganate (\[KMn{{O}_{4}}\]) gives potassium manganate (\[{{K}_{2}}Mn{{O}_{4}}\]). The oxidation state of manganese in \[{{K}_{2}}Mn{{O}_{4}}\] is +6.
Complete step by step solution:
-The structure of the potassium permanganate is as follows.

-The reaction of heating of potassium permanganate is as follows.
\[KMn{{O}_{4}}\xrightarrow{\Delta }{{K}_{2}}Mn{{O}_{4}}+Mn{{O}_{2}}+{{O}_{2}}\]
-On heating, potassium permanganate gives potassium manganate, Manganese dioxide and oxygen as the products.
-Coming to given options, option A Hydrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no hydrogen. So, on heating potassium permanganate, hydrogen gas is not going to form as one of the product. Hence option A is wrong.
-Coming option B, Oxygen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is a presence of oxygen. So, on heating potassium permanganate, oxygen gas is going to form as one of the product. Hence option B is correct.
-Coming to option C, Nitrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no nitrogen. So, on heating potassium permanganate, Nitrogen gas is not going to form as one of the product. Hence option C is wrong.
-Coming to option D, Methane. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]) there is no Carbon and hydrogen. So, on heating potassium permanganate, Methane gas is not going to form as one of the product. Hence option D is wrong.
-Therefore on heating potassium permanganate, oxygen gas is going to form as one of the product.
So, the correct option is (B).
Note: The oxidation state of Manganese in potassium permanganate (\[KMn{{O}_{4}}\]) is +7. On heating potassium permanganate (\[KMn{{O}_{4}}\]) gives potassium manganate (\[{{K}_{2}}Mn{{O}_{4}}\]). The oxidation state of manganese in \[{{K}_{2}}Mn{{O}_{4}}\] is +6.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




