
The formula of silver phosphate is:
A. \[AgP{O_4}\]
B. \[A{g_3}P{O_4}\]
C. \[A{g_2}{(P{O_4})_3}\]
D. \[A{g_2}P{O_4}\]
Answer
618k+ views
Hint: In this question we will use the concept of chemical formula and structure of a compound. We will observe the chemical properties of the elements involved in the compound and then by making the suitable structure, we can easily find out the chemical formula of the compound.
Complete answer:
Elements present in silver phosphate are: silver(Ag), oxygen(O) and phosphorus(P).
Silver phosphate, also known as argentous phosphate or silver orthophosphate, is an inorganic salt used as stained or light sensitive substance for revealing in photography.
A phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid \[{H_3}P{O_4}\].
This salt is formed by three silver cations ${(Ag)^ + }$and one phosphate anion ${(P{O_4})^{3 - }}$which forms a cubic crystal structure. Its chemical structure can be written as below, in the common representations used for organic molecules.
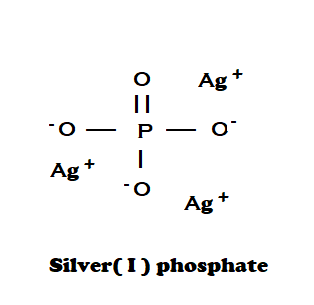
The chemical formula of silver phosphate is \[A{g_3}P{O_4}\].
Silver phosphate is formed as a yellow precipitate when it is reacted with silver nitrate and the salt orthophosphate. The product obtained is not soluble in water, thus it can be filtered and recovered as a pure solid.
$AgN{O_3} + P{O_4}^{3 - } \to A{g_3}P{O_4}(s)$
Hence, the correct answer is option (B).
Note: Whenever we are asked these types of questions, first we have to remember some basic points of formation of the chemical formula of a compound. Then we will find out the elements that are involved in the compound. Observing their properties we have to make the chemical structure of the compound and through this we will get the chemical formula.
Complete answer:
Elements present in silver phosphate are: silver(Ag), oxygen(O) and phosphorus(P).
Silver phosphate, also known as argentous phosphate or silver orthophosphate, is an inorganic salt used as stained or light sensitive substance for revealing in photography.
A phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid \[{H_3}P{O_4}\].
This salt is formed by three silver cations ${(Ag)^ + }$and one phosphate anion ${(P{O_4})^{3 - }}$which forms a cubic crystal structure. Its chemical structure can be written as below, in the common representations used for organic molecules.

The chemical formula of silver phosphate is \[A{g_3}P{O_4}\].
Silver phosphate is formed as a yellow precipitate when it is reacted with silver nitrate and the salt orthophosphate. The product obtained is not soluble in water, thus it can be filtered and recovered as a pure solid.
$AgN{O_3} + P{O_4}^{3 - } \to A{g_3}P{O_4}(s)$
Hence, the correct answer is option (B).
Note: Whenever we are asked these types of questions, first we have to remember some basic points of formation of the chemical formula of a compound. Then we will find out the elements that are involved in the compound. Observing their properties we have to make the chemical structure of the compound and through this we will get the chemical formula.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Write a letter to the editor of a newspaper explaining class 10 english CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?




