
The formula of one molecule of phosphorus is:
\[A.\;\;\;\;\;P \\
B.\;\;\;\;\;{P_2} \\
C.\;\;\;\;\;{P_4} \\
D.\;\;\;\;\;{P_8} \]
Answer
513.3k+ views
Hint :Phosphorus forms only three bonds. It exists as tetra-atomic. The size of phosphorus is very large because of which the p-orbitals which usually overlap each other are unable to overlap in phosphorus to make a pie bond. But phosphorus can show catenation.
Complete Step By Step Answer:
The total number of electrons in phosphorus is$15$. The outer orbital consists of $5$electrons in phosphorus, and there are $10$electrons in the inner orbitals so the difference between the nucleus and the outer $5$electrons is big. So the outer $5$electrons are unable to form strong triple bonds. The outer orbital of phosphorus contains a large space because the electrons are far apart from each other and they need to complete their octet state so to become stable and complete its octet phosphorus gets bonded to three other atoms of phosphorus. And so phosphorus becomes tetra atomic which means its atomicity is$4$. Hence phosphorus exists as\[{P_4}\]. Therefore the formula of one molecule of phosphorus is \[{P_4}\]
\[{P_4}\] is also known as white phosphorus. The single bonds between the atoms are very weak; this makes phosphorus a very reactive molecule. It is waxy solid and very poisonous. \[{P_4}\] needs to be stored underwater because in the presence of air it burns vigorously. When it burns in the air and gives diphosphorus pentoxide. It smells like garlic. In both solid and vapor states the phosphorus molecule exists as\[{P_4}\]. The structure of \[{P_4}\]is shown below:
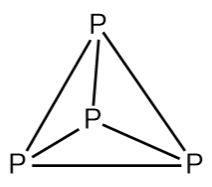
Hence from the above information, we can say that formula of one molecule of phosphorus is \[{P_4}\]
Therefore the correct option is \[C.\;\;\;\;\;{P_4}\].
Note :
Phosphorus has three allotropes white phosphorus, red phosphorus, and black phosphorus. Both white and red phosphorus exist as \[{P_4}\] molecules whereas black phosphorus exists in two different forms $\alpha - black - P$ and$\beta - black - P$. Among the three white phosphorus is highly reactive.
Complete Step By Step Answer:
The total number of electrons in phosphorus is$15$. The outer orbital consists of $5$electrons in phosphorus, and there are $10$electrons in the inner orbitals so the difference between the nucleus and the outer $5$electrons is big. So the outer $5$electrons are unable to form strong triple bonds. The outer orbital of phosphorus contains a large space because the electrons are far apart from each other and they need to complete their octet state so to become stable and complete its octet phosphorus gets bonded to three other atoms of phosphorus. And so phosphorus becomes tetra atomic which means its atomicity is$4$. Hence phosphorus exists as\[{P_4}\]. Therefore the formula of one molecule of phosphorus is \[{P_4}\]
\[{P_4}\] is also known as white phosphorus. The single bonds between the atoms are very weak; this makes phosphorus a very reactive molecule. It is waxy solid and very poisonous. \[{P_4}\] needs to be stored underwater because in the presence of air it burns vigorously. When it burns in the air and gives diphosphorus pentoxide. It smells like garlic. In both solid and vapor states the phosphorus molecule exists as\[{P_4}\]. The structure of \[{P_4}\]is shown below:

Hence from the above information, we can say that formula of one molecule of phosphorus is \[{P_4}\]
Therefore the correct option is \[C.\;\;\;\;\;{P_4}\].
Note :
Phosphorus has three allotropes white phosphorus, red phosphorus, and black phosphorus. Both white and red phosphorus exist as \[{P_4}\] molecules whereas black phosphorus exists in two different forms $\alpha - black - P$ and$\beta - black - P$. Among the three white phosphorus is highly reactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




