
The formation of benzene from acetylene is :
a.Dimerization
b.Tetramerization
c.Dimerization
d.Condensation.
Answer
469.2k+ views
Hint: Three out of the four given options are the types or categories of polymerization reactions. A polymerization reaction mainly involves multiple monomers together in the form of a long chain or a ring. The classification of polymerization depends on the number of monomers being used.
Complete step by step answer:
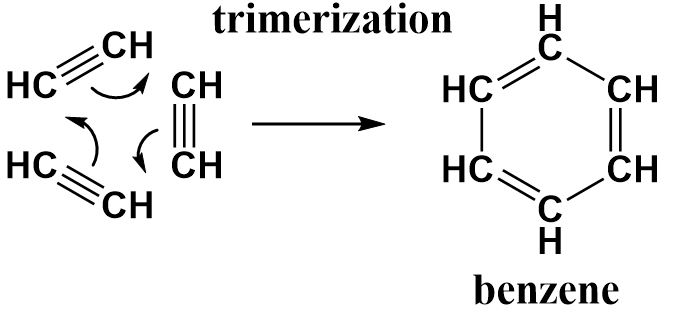
Acetylene is an unsaturated organic compound containing two carbon atoms that are strongly bonded to one another through triple bonds.
Benzene is an aromatic compound consisting of six carbon atoms bonded to each in the form of a six-membered ring (hexagonal since all bond distances are equal) with alternative double bonds that keep delocalizing throughout the ring.
The conversion of acetylene requires three molecules of acetylene to produce a single benzene ring. The triple bonds in each acetylene molecule open up to form a single bond with the carbon atom of an adjacent acetylene molecule. All three of them are arranged in a cyclic manner to obtain a ring structure consisting of alternating double bonds similar to benzene.
Each acetylene molecule acts as a monomer and therefore benzene can be called a trimer of acetylene.
Hence, this reaction can be called a trimerization reaction and the correct option is (a).
Note:
The condensation reaction is said to occur when two or more monomers combine to form a new covalent bond in which a small organic or inorganic molecule is removed like water or ammonia. Acetylene molecules do not eliminate any such molecule and simply polymerize to give benzene.
Complete step by step answer:
Acetylene is an unsaturated organic compound containing two carbon atoms that are strongly bonded to one another through triple bonds.
Benzene is an aromatic compound consisting of six carbon atoms bonded to each in the form of a six-membered ring (hexagonal since all bond distances are equal) with alternative double bonds that keep delocalizing throughout the ring.
The conversion of acetylene requires three molecules of acetylene to produce a single benzene ring. The triple bonds in each acetylene molecule open up to form a single bond with the carbon atom of an adjacent acetylene molecule. All three of them are arranged in a cyclic manner to obtain a ring structure consisting of alternating double bonds similar to benzene.
Each acetylene molecule acts as a monomer and therefore benzene can be called a trimer of acetylene.
Hence, this reaction can be called a trimerization reaction and the correct option is (a).

Note:
The condensation reaction is said to occur when two or more monomers combine to form a new covalent bond in which a small organic or inorganic molecule is removed like water or ammonia. Acetylene molecules do not eliminate any such molecule and simply polymerize to give benzene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




