
The following units exist in chain silicates: -
A. ${(Si{O_5})_n}^{2n - }$
B. ${\left( {Si{O_3}} \right)_n}^{3 - }$
C. ${\left( {Si{O_3}} \right)_n}^{2n - }$
D. ${\left( {Si{O_6}} \right)_n}^{2 - }$
Answer
569.7k+ views
Hint:We know that Silicates are nothing but coordinate compounds which are made up of large anions arranged about small cations. So, the dimensions of the structure of silicates will be controlled by anions. The basic building block of all silicate minerals is the ${[Si{O_4}]^{4 - }}$ tetrahedron. Silicate minerals containing chains are termed as inosilicates. There are two types of chain silicates which are present which are single chain silicates and double chain silicates.
Complete answer:
Chain silicates also known as pyroxenes contains ${\left( {Si{O_3}} \right)_n}^{2n - }$ ions which are formed by linking $'n'$ number of ${[Si{O_4}]^{4 - }}$ tetrahedral units linearly. Each unit shares two oxygen atoms with other units.
Here we see that Silica undergoes hybridisation to form $s{p^3}$ hybridisation. In this hybridisation four unpaired electrons are available. Oxygen binds with these unpaired electrons and hence forms four covalent silica-oxygen bonds. Since the hybridisation of silica oxide is $s{p^3}$ so it will form a tetrahedral geometry with each oxygen atom forming the vertex of the tetrahedron. Thus, the silica to oxygen ratio would be $1:4$ .
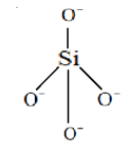
We will now draw the structure of ${[Si{O_4}]^{4 - }}$
Let us see some examples of chain silicates: -
Spodumene - $LiAl{(Si{O_3})_2}$
Diopside - $CaMg(Si{O_3}){ _2}$
Wollastonite- $C{a_3}{\left( {Si{O_3}} \right)_3}$
It is clear from the above text that the unit present in chain silicates is ${\left( {Si{O_3}} \right)_n}^{2n - }$.
Therefore the correct option will be Option C.
Additional information:
Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements. The oxygen to silicate ratio helps to determine the degree of polymerization. The greater the degree of polymerization, the lower will be the silicate to oxygen ratio.
Note:
Since the silicon ion has a charge of $ + 4$ and each of the four oxygen ions has a charge of $ - 2$, the silica tetrahedron has a net charge of $ - 4$ . We also see that the formula of cyclic silicates as well as chain silicates is ${\left( {Si{O_3}} \right)_n}^{2n - }$ . Hence, they are considered as oligomers of the unknown $Si{O_3}^{2 - }$ ions. Since the silicon ion has a charge of $ + 4$ and each of the four oxygen ions has a charge of $ - 2$, the silica tetrahedron has a net charge of $ - 4$ .
Complete answer:
Chain silicates also known as pyroxenes contains ${\left( {Si{O_3}} \right)_n}^{2n - }$ ions which are formed by linking $'n'$ number of ${[Si{O_4}]^{4 - }}$ tetrahedral units linearly. Each unit shares two oxygen atoms with other units.
Here we see that Silica undergoes hybridisation to form $s{p^3}$ hybridisation. In this hybridisation four unpaired electrons are available. Oxygen binds with these unpaired electrons and hence forms four covalent silica-oxygen bonds. Since the hybridisation of silica oxide is $s{p^3}$ so it will form a tetrahedral geometry with each oxygen atom forming the vertex of the tetrahedron. Thus, the silica to oxygen ratio would be $1:4$ .
We will now draw the structure of ${[Si{O_4}]^{4 - }}$

Let us see some examples of chain silicates: -
Spodumene - $LiAl{(Si{O_3})_2}$
Diopside - $CaMg(Si{O_3}){ _2}$
Wollastonite- $C{a_3}{\left( {Si{O_3}} \right)_3}$
It is clear from the above text that the unit present in chain silicates is ${\left( {Si{O_3}} \right)_n}^{2n - }$.
Therefore the correct option will be Option C.
Additional information:
Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements. The oxygen to silicate ratio helps to determine the degree of polymerization. The greater the degree of polymerization, the lower will be the silicate to oxygen ratio.
Note:
Since the silicon ion has a charge of $ + 4$ and each of the four oxygen ions has a charge of $ - 2$, the silica tetrahedron has a net charge of $ - 4$ . We also see that the formula of cyclic silicates as well as chain silicates is ${\left( {Si{O_3}} \right)_n}^{2n - }$ . Hence, they are considered as oligomers of the unknown $Si{O_3}^{2 - }$ ions. Since the silicon ion has a charge of $ + 4$ and each of the four oxygen ions has a charge of $ - 2$, the silica tetrahedron has a net charge of $ - 4$ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




