
The following three objects (1) a metal tray, (2) a block of wood, and (3) a woolen cap are left in a closed room overnight. The next day the temperature of each is recorded as $T_1$, $T_2$, and $T_3$ respectively. The likely situation is
A) $\mathop T\nolimits_1 = \mathop T\nolimits_2 = \mathop T\nolimits_3 $
B) $\mathop T\nolimits_3 > \mathop T\nolimits_2 > \mathop T\nolimits_1 $
C) $\mathop T\nolimits_3 = \mathop T\nolimits_2 > \mathop T\nolimits_1 $
D) $\mathop T\nolimits_3 > \mathop T\nolimits_2 = \mathop T\nolimits_1 $
Answer
585.3k+ views
Hint: equilibrium constants are not changed if you change the concentrations of things present in the equilibrium. The only thing that changes an equilibrium constant is a change of temperature, the position of equilibrium is changed if you change the concentration of something present in the mixture.
Complete step by step answer:
Zeroth law of thermodynamics:
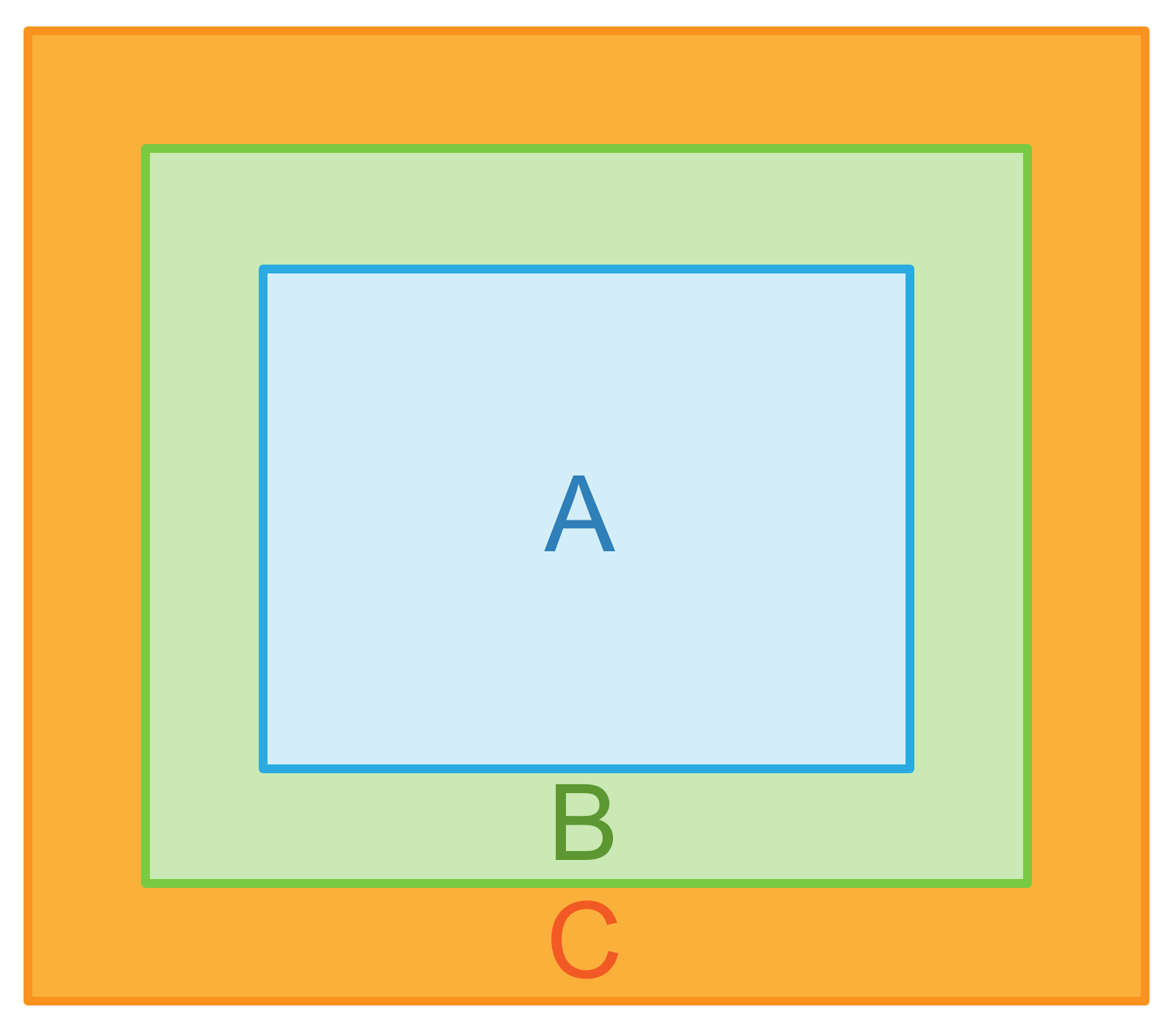
Consider three thermodynamic systems A, B, and C. When the system A is in thermal equilibrium with system B and also separately in thermal equilibrium with system C then the system B and C are said to be thermal equilibrium with each other. In other words, we can say that the system that is in the thermal equilibrium exists at the same temperature.
The temperature of each three-body will remain constant because they have enough time to reach thermal equilibrium, and as per zeroth law of thermodynamics
$\mathop T\nolimits_1 = \mathop T\nolimits_2 = \mathop T\nolimits_3 $
Sometimes we are confused because we touch the metal body and wooden block. We felt that the metal body has a slightly low temperature but we didn't because the thermal conductivity of metal is very good. The rate of heat transfer from the body to our hand is faster in the metal body case and vice-versa in the wooden blocks so we felt it’s a higher temperature.
As all the three objects are placed inside a closed room. So, heat transfer through convection will be zero. As no heat is entering the room from outside, the objects or room air at higher temperatures will lose heat through radiation and conduction to an object or air at lower temperatures. Hence, objects will reach a state of thermal equilibrium with each other and room air. So, the temperature of all three objects will be equal.
So, the correct answer is option (A).
Note:
Heat energy always flows from a higher temperature body to a lower temperature body.
A calorie is the most commonly used unit of heat. Heat energy is transferred from one body to another when there is a temperature difference. Energy travels from a hotter body to a cooler body.
Complete step by step answer:
Zeroth law of thermodynamics:

Consider three thermodynamic systems A, B, and C. When the system A is in thermal equilibrium with system B and also separately in thermal equilibrium with system C then the system B and C are said to be thermal equilibrium with each other. In other words, we can say that the system that is in the thermal equilibrium exists at the same temperature.
The temperature of each three-body will remain constant because they have enough time to reach thermal equilibrium, and as per zeroth law of thermodynamics
$\mathop T\nolimits_1 = \mathop T\nolimits_2 = \mathop T\nolimits_3 $
Sometimes we are confused because we touch the metal body and wooden block. We felt that the metal body has a slightly low temperature but we didn't because the thermal conductivity of metal is very good. The rate of heat transfer from the body to our hand is faster in the metal body case and vice-versa in the wooden blocks so we felt it’s a higher temperature.
As all the three objects are placed inside a closed room. So, heat transfer through convection will be zero. As no heat is entering the room from outside, the objects or room air at higher temperatures will lose heat through radiation and conduction to an object or air at lower temperatures. Hence, objects will reach a state of thermal equilibrium with each other and room air. So, the temperature of all three objects will be equal.
So, the correct answer is option (A).
Note:
Heat energy always flows from a higher temperature body to a lower temperature body.
A calorie is the most commonly used unit of heat. Heat energy is transferred from one body to another when there is a temperature difference. Energy travels from a hotter body to a cooler body.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




