
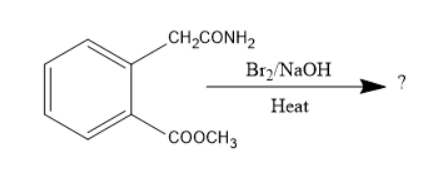
The following sequence of reactions gives:
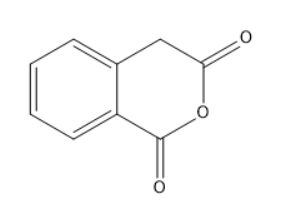
A.
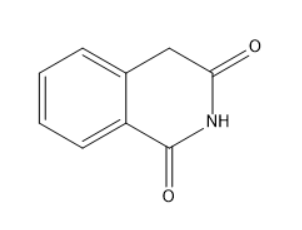
B.
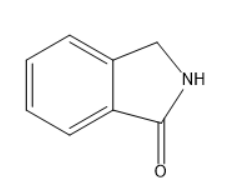
C.
D.
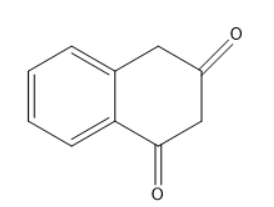





Answer
478.5k+ views
Hint: Amides are the compounds consisting of \[ - CON{H_2}\] functional group. Amides undergo Hoffmann bromination when treated with bromine in presence of sodium hydroxide to form primary amines. The primary amine and ester presence in the same molecule loses alcohol and undergoes cyclization.
Complete answer:
Given compound is a derivative of benzene. The substituents were ethanamide and methyl methanoate. These two groups were at ortho position to each other.
When the compound consisting of amide treated with bromine in presence of alkali like sodium hydroxide undergoes Hoffmann bromination to form primary amine. Thus, the amide group converts into a primary amine. The chemical reaction involved will be as follows:
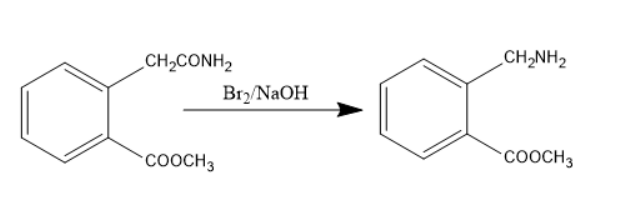
The compound formed has primary amine and ester groups. When this compound is heated, it loses the methanol molecule, the methoxy group can be lost from the ester group and hydrogen from the amine group and forms a cyclized product.
The chemical reaction involved will be as follows:
Thus, the cyclic product will be a lactam. The cyclic amides are known as lactam.
The name of the product will be isoindolin- \[1\]-one. It belongs to the indole family as nitrogen is involved in a cyclic structure.
The final compound matches with the structure in option C.
Option C is the correct one.
Note:
Hoffmann bromination is an important organic reaction. It is one of the methods to prepare primary amines from amides. As primary amines are more basic and can easily lose a proton, whereas the ester loses the methoxy group. On heating, methanol will be eliminated.
Complete answer:
Given compound is a derivative of benzene. The substituents were ethanamide and methyl methanoate. These two groups were at ortho position to each other.
When the compound consisting of amide treated with bromine in presence of alkali like sodium hydroxide undergoes Hoffmann bromination to form primary amine. Thus, the amide group converts into a primary amine. The chemical reaction involved will be as follows:

The compound formed has primary amine and ester groups. When this compound is heated, it loses the methanol molecule, the methoxy group can be lost from the ester group and hydrogen from the amine group and forms a cyclized product.
The chemical reaction involved will be as follows:
Thus, the cyclic product will be a lactam. The cyclic amides are known as lactam.
The name of the product will be isoindolin- \[1\]-one. It belongs to the indole family as nitrogen is involved in a cyclic structure.
The final compound matches with the structure in option C.
Option C is the correct one.
Note:
Hoffmann bromination is an important organic reaction. It is one of the methods to prepare primary amines from amides. As primary amines are more basic and can easily lose a proton, whereas the ester loses the methoxy group. On heating, methanol will be eliminated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




