
The five d-orbitals are designated as ${{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}$ . Choose the correct statement from the given options.
[A] The shape of the first three orbits is similar but that of the fourth and fifth orbitals are different.
[B] The shape of all the five d-orbitals are similar.
[C] The shape of the first four orbitals are similar and that of the fifth orbital is different.
[D] The shapes of all the five d-orbitals are different.
Answer
595.2k+ views
Hint: d-orbitals have a double dumbbell shape. The subscripts in the designation of the d-orbitals depict the orientation of the orbitals along the axes. Their shape is often known as clover like.
Complete step by step answer:
We know that the d sublevel has 5 energy orbitals. The maximum number of electrons in one orbital is 2 and thus the 5 orbitals can fit in maximum 10 electrons.
The five d-orbitals are designated as ${{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}$ depending upon the plane they are lying upon. The d-orbitals have a double dumbbell shape and thus they are named differently depending upon how the double dumbbell is oriented in the axes.
Now, let us discuss their shapes and their orientation-
- The orbital lying in the xy-plane is designated as ${{d}_{xy}}$.
- The orbital lying in the yz-plane is designated as ${{d}_{yz}}$.
- The orbital lying in the xy-plane is designated as ${{d}_{zx}}$.
- The ${{d}_{{{x}^{2}}-{{y}^{2}}}}$ orbital lies on the x-y axes.
- The ${{d}_{{{z}^{2}}}}$ orbital has a single dumbbell shape and it lies along the z-axis. There is a ring of negative charge around it along the xy-plane.
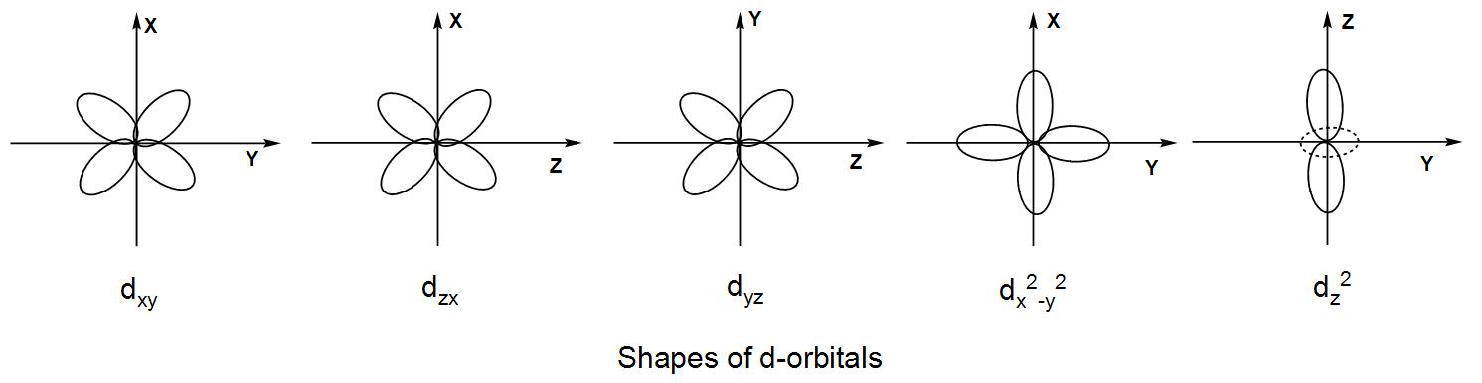
The four d-orbitals have similar double dumbbell shape just in the fourth orbital the structure is oriented along the axes instead of a plane and the fifth orbital has a single dumbbell shape with a ring around it and it is often called a donut shaped orbital.
We can understand from the above discussion that all the orbitals have different orientations but the four d-orbitals have a similar shape and the fifth one has a different shape compared to the others.
So, the correct answer is “Option C”.
Note: The orbitals differ in shape due to having different probabilities of finding electrons around the nucleus. The point where there is no possibility of finding a nucleus is known as a node. In d-orbitals, there are 2 nodal planes. This is due to their double dumbbell shape.
Complete step by step answer:
We know that the d sublevel has 5 energy orbitals. The maximum number of electrons in one orbital is 2 and thus the 5 orbitals can fit in maximum 10 electrons.
The five d-orbitals are designated as ${{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}$ depending upon the plane they are lying upon. The d-orbitals have a double dumbbell shape and thus they are named differently depending upon how the double dumbbell is oriented in the axes.
Now, let us discuss their shapes and their orientation-
- The orbital lying in the xy-plane is designated as ${{d}_{xy}}$.
- The orbital lying in the yz-plane is designated as ${{d}_{yz}}$.
- The orbital lying in the xy-plane is designated as ${{d}_{zx}}$.
- The ${{d}_{{{x}^{2}}-{{y}^{2}}}}$ orbital lies on the x-y axes.
- The ${{d}_{{{z}^{2}}}}$ orbital has a single dumbbell shape and it lies along the z-axis. There is a ring of negative charge around it along the xy-plane.

The four d-orbitals have similar double dumbbell shape just in the fourth orbital the structure is oriented along the axes instead of a plane and the fifth orbital has a single dumbbell shape with a ring around it and it is often called a donut shaped orbital.
We can understand from the above discussion that all the orbitals have different orientations but the four d-orbitals have a similar shape and the fifth one has a different shape compared to the others.
So, the correct answer is “Option C”.
Note: The orbitals differ in shape due to having different probabilities of finding electrons around the nucleus. The point where there is no possibility of finding a nucleus is known as a node. In d-orbitals, there are 2 nodal planes. This is due to their double dumbbell shape.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




