
The figure shows the variation of specific heat capacity $(C)$ of a solid as a function of temperature $(T)$. The temperature is increased continuously from $0$ to $500\;K$ at a constant rate. Ignoring any volume change, the following statements are correct to a reasonable approximation:
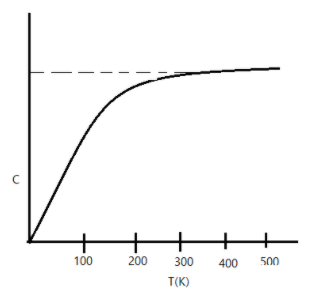
A. the rate at which heat is absorbed in the range $0-100\;K$ varies linearly with temperature $T$.
B. heat absorbed in increasing the temperature from $0-100\;K$ is less than the heat required for increasing the temperature from $400-500\;K$
C. there is no change in the rate of hat absorption in range$400-500\;K$
D. the rate of heat absorption increase in the range $200-300\;K$

Answer
580.2k+ views
Hint: Heat transfer coefficient is proportionality constant between the heat flux and the direction of change in heat. Thus clearly it depends on temperature. Here, we are given the graph, using which we can find the answers.
Complete answer:
We know that heat is a form of energy which can be transferred from one body to another. Also, $C=\dfrac{dU}{dT}$, where $C$ is the heat capacitance, $U$ is the internal energy of the system, and $T$ is the temperature.
Since $dQ=mCdT$, where $dQ$ is the heat absorbed or released, $m$ is the mass of the substance and $dT$ is the change in temperature.
Thus clearly, we can say the rate of change of heat is proportional to the temperature, irrespective of the heat capacity. Thus option A is correct.
As the value of $C$ is more from $400-500\;K$ than $0-100\;K$, we can say that heat is absorbed to reach $400-500\;K$. Thus option B is also correct.
The same is true for$200-300\;K$, thus option D is also correct.
Since the value of $C$ remains constant after $400-500\;K$, we can say that there is no change in heat. Thus option C is also correct
Hence clearly, A. the rate at which heat is absorbed in the range $0-100\;K$ varies linearly with temperature $T$,B. heat absorbed in increasing the temperature from $0-100\;K$ is less than the heat required for increasing the temperature from $400-500\;K$, C. there is no change in the rate of hat absorption in range $400-500\;K$ and D. the rate of heat absorption increase in the range $200-300\;K$ are the correct answers.
Note:
Thermal conductivity is the transfer of heat energy due to random motion of the molecules in given material. And it is the inverse of thermal resistivity. While heat transfer coefficient gives the relationship between the heat transferred across the boundary of a given body.
Complete answer:
We know that heat is a form of energy which can be transferred from one body to another. Also, $C=\dfrac{dU}{dT}$, where $C$ is the heat capacitance, $U$ is the internal energy of the system, and $T$ is the temperature.
Since $dQ=mCdT$, where $dQ$ is the heat absorbed or released, $m$ is the mass of the substance and $dT$ is the change in temperature.
Thus clearly, we can say the rate of change of heat is proportional to the temperature, irrespective of the heat capacity. Thus option A is correct.
As the value of $C$ is more from $400-500\;K$ than $0-100\;K$, we can say that heat is absorbed to reach $400-500\;K$. Thus option B is also correct.
The same is true for$200-300\;K$, thus option D is also correct.
Since the value of $C$ remains constant after $400-500\;K$, we can say that there is no change in heat. Thus option C is also correct
Hence clearly, A. the rate at which heat is absorbed in the range $0-100\;K$ varies linearly with temperature $T$,B. heat absorbed in increasing the temperature from $0-100\;K$ is less than the heat required for increasing the temperature from $400-500\;K$, C. there is no change in the rate of hat absorption in range $400-500\;K$ and D. the rate of heat absorption increase in the range $200-300\;K$ are the correct answers.
Note:
Thermal conductivity is the transfer of heat energy due to random motion of the molecules in given material. And it is the inverse of thermal resistivity. While heat transfer coefficient gives the relationship between the heat transferred across the boundary of a given body.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




