
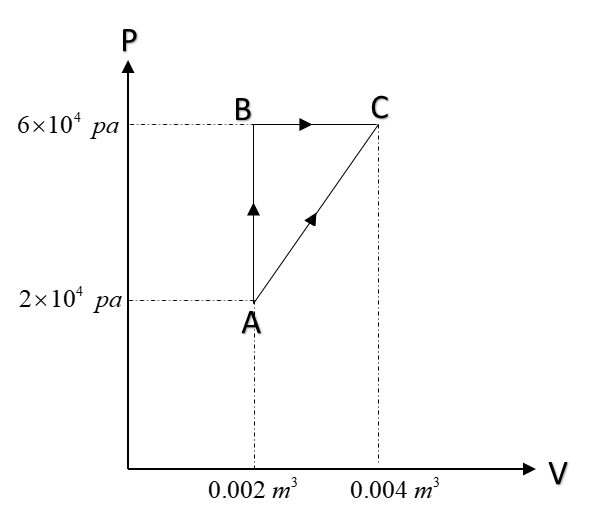
The figure below shows two paths that may be taken by a gas to go from a state A to state C.
In process AB, 400J of heat is added to the system and in process BC, 100J of heat is added to the system. The heat absorbed by the system in the process AC will be:
A. 460 J
B. 300 J
C. 380 J
D. 500 J

Answer
571.8k+ views
Hint: First law of thermodynamics is basically the law of conservation of energy which states that energy can neither be created nor be destroyed, but can transform from one form to another. In the language of thermodynamics, we can say that if the gas contains a certain amount of energy, it can be used to do work on a system.
Formula used:
$dQ = dU+dW, \ dW = \int PdV$(area under PV curve)
Complete step-by-step answer:
We know that the heat supplied to the system goes in either increasing internal energy of the gas or doing work or both (depends upon the process).
Also, $dW = PdV$
Now, we are asked about the process AC which is not a generalized process. But AB and BC are general processes known to us. Thus we shall proceed by working on AB and BC and hence we can collect data about AC.
Now, for AB, we can see that the process is isochoric.
So $dV = 0 \ and \ W_{AB} =0$
Now, given that in process AB, heat supplied = +400J
Thus $Q_{AB} = 400 J$
Hence from first law of thermodynamics, $U_{AB} = 400-0=400J$
Now, for process BC,
$W = \int PdV = P(V_C - V_B) = 6\times 10^4 ( 0.004-0.002) = 120J$
Or $W_{BC} = 120J$
Also, given $Q_{BC} = 100J$
Hence from first law of thermodynamics, $U_{BC} = 100-120=-20J$
Now, as we know that internal energy is a state function and hence depends upon the initial and final state of the system. Thus for a complete cycle, $\Delta U =0$
$\implies U_{AB} + U_{BC} + U_{CA} = 0$
So, $U_{CA} = -(U_{AB} + U_{BC}) = -(400 -20) = -380J$
Also $U_{CA} = -U_{AC} = 380 J$
Now, we are supposed to find heat absorbed during AC, thus $Q_{AC} = U_{AC} + W_{AC}$
Now, for $W_{AC} = area\ under\ AC = \dfrac12\left(2\times 10^4+6\times 10^4\right)\times 0.002 = 80 J$
Thus, $Q_{AC} = U_{AC} + W_{AC}$
$Q_{AC} = 380 + 80 = 460 J$
Hence option A. is correct.
So, the correct answer is “Option A”.
Note: Isobaric process- When the gas undergoes a process in which its pressure remains constant throughout the process, is called isobaric pressure.
Isochoric process- When the gas undergoes a process in which its volume remains constant throughout the process, is called isochoric pressure.
Formula used:
$dQ = dU+dW, \ dW = \int PdV$(area under PV curve)
Complete step-by-step answer:
We know that the heat supplied to the system goes in either increasing internal energy of the gas or doing work or both (depends upon the process).
Also, $dW = PdV$
Now, we are asked about the process AC which is not a generalized process. But AB and BC are general processes known to us. Thus we shall proceed by working on AB and BC and hence we can collect data about AC.
Now, for AB, we can see that the process is isochoric.
So $dV = 0 \ and \ W_{AB} =0$
Now, given that in process AB, heat supplied = +400J
Thus $Q_{AB} = 400 J$
Hence from first law of thermodynamics, $U_{AB} = 400-0=400J$
Now, for process BC,
$W = \int PdV = P(V_C - V_B) = 6\times 10^4 ( 0.004-0.002) = 120J$
Or $W_{BC} = 120J$
Also, given $Q_{BC} = 100J$
Hence from first law of thermodynamics, $U_{BC} = 100-120=-20J$
Now, as we know that internal energy is a state function and hence depends upon the initial and final state of the system. Thus for a complete cycle, $\Delta U =0$
$\implies U_{AB} + U_{BC} + U_{CA} = 0$
So, $U_{CA} = -(U_{AB} + U_{BC}) = -(400 -20) = -380J$
Also $U_{CA} = -U_{AC} = 380 J$
Now, we are supposed to find heat absorbed during AC, thus $Q_{AC} = U_{AC} + W_{AC}$
Now, for $W_{AC} = area\ under\ AC = \dfrac12\left(2\times 10^4+6\times 10^4\right)\times 0.002 = 80 J$
Thus, $Q_{AC} = U_{AC} + W_{AC}$
$Q_{AC} = 380 + 80 = 460 J$
Hence option A. is correct.
So, the correct answer is “Option A”.
Note: Isobaric process- When the gas undergoes a process in which its pressure remains constant throughout the process, is called isobaric pressure.
Isochoric process- When the gas undergoes a process in which its volume remains constant throughout the process, is called isochoric pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




