
The figure below shows four isotropic solids heaving positive coefficient of thermal expansion. A student predicts that by heating the solid following things can happen. Mark true (T) or False (F) for comments made by the student.
(i) The angle $\alpha $ in figure $\left( 1 \right)$ will not change.
(ii) The length of line in figure $\left( 2 \right)$ will decreases
(iii) The radius of inner hole will decreases
(iv) The distance AB will increase.
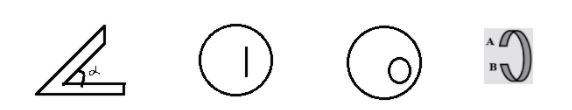
A. T F F T.
B. F T T F.
C. T T T T.
D. F F T F.
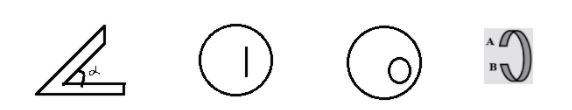
Answer
594.9k+ views
Hint: We can approach this problem with the concept of thermal expansion of solids. When a solid is heated, its atoms vibrate about their fixed positions. When heat is applied on solids, it can expand according to dimension such as it expands in its lengths, area or volume. The expansion in the length is called linear expansion, and if length and breadth expands, it is known as area expansion and if a cube is heated, expansion happens in its entire volume and it is called volume expansion.
Complete step-by-step answer:
Solids expand on heating and contract on cooling. Thermal expansion can be defined as the change in the dimensions of material on changing the temperature.
(i) This statement is TRUE because when the heat is applied expansion will be uniform in its all dimensions so the angle remains the same as it was before.
(ii) For the second one, also the expansion will be in all dimensions so this statement is false and the length of line will also increase.
(iii) Increases in temperature also increase the separation between the particles .So it is not possible that the radius of inner circles will decrease. According to this , the third statement is false and the radius of the inner circle will increase.
(iv) This is true because on the temperature increase there will be an increase in the distance AB.
So, the correct answer is “Option A”.
Note: When the temperature changes, the coefficient of thermal expansion is responsible for how much the increase in the dimensions of materials can be achieved. Larger the coefficient of material, more it will expand.
Complete step-by-step answer:
Solids expand on heating and contract on cooling. Thermal expansion can be defined as the change in the dimensions of material on changing the temperature.
(i) This statement is TRUE because when the heat is applied expansion will be uniform in its all dimensions so the angle remains the same as it was before.
(ii) For the second one, also the expansion will be in all dimensions so this statement is false and the length of line will also increase.
(iii) Increases in temperature also increase the separation between the particles .So it is not possible that the radius of inner circles will decrease. According to this , the third statement is false and the radius of the inner circle will increase.
(iv) This is true because on the temperature increase there will be an increase in the distance AB.
So, the correct answer is “Option A”.
Note: When the temperature changes, the coefficient of thermal expansion is responsible for how much the increase in the dimensions of materials can be achieved. Larger the coefficient of material, more it will expand.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




