
The explosive TNT is made from:
A. Thorium
B. Phthalic acid
C. Toluene
D. Tin
Answer
534.3k+ views
Hint: An explosive compound is that which is capable of a reaction to produce a large amount of gas, heat and energy at a very high pressure and temperature to cause environmental damage. Some examples of explosives are, dynamite, nitroglycerin, etc.
Complete answer:
Explosives are chemical compounds that undergo a series of rapid oxidation reactions to liberate large amounts of gases. The temperature and pressure of these gases contribute to the energy of an explosion.
The explosive TNT stands for trinitrotoluene, or its IUPAC name is 2,4,6-trinitrotoluene. TNT is a great explosive because of the carbon, nitrogen and oxygen it contains. After the oxidation of this compound, gases formed are, carbon monoxide, $CO$, carbon dioxide, $C{{O}_{2}}$, and nitrogen gas${{N}_{2}}$, which are very stable and releases high amount of energy.
As the name suggests TNT, trinitrotoluene, is made from toluene. This toluene is treated with nitric and sulphuric acid mixtures to obtain TNT. The structure of TNT is as follows:
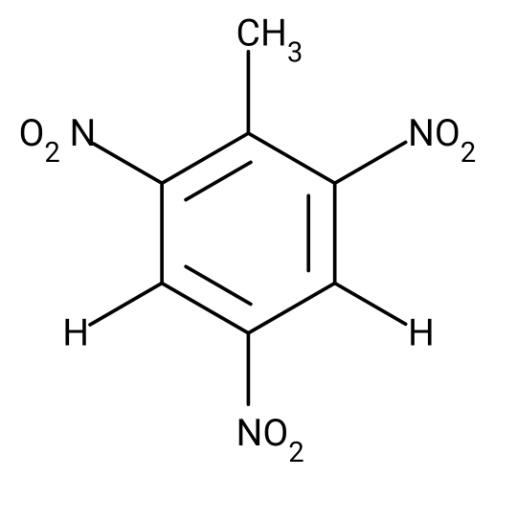
Clearly, the structure of TNT does not have thorium, phthalic acid or tin, therefore, it is made of toluene.
Hence, the explosive TNT is made from toluene.
Note:
TNT is used as an explosive for bombs, grenades and in military uses, also for collapsing buildings. The gases that are evolved are considered as very poisonous and can lead to serious injuries and illnesses in humans. The explosion creates a high amount of shock waves that result in mass deterioration.
Complete answer:
Explosives are chemical compounds that undergo a series of rapid oxidation reactions to liberate large amounts of gases. The temperature and pressure of these gases contribute to the energy of an explosion.
The explosive TNT stands for trinitrotoluene, or its IUPAC name is 2,4,6-trinitrotoluene. TNT is a great explosive because of the carbon, nitrogen and oxygen it contains. After the oxidation of this compound, gases formed are, carbon monoxide, $CO$, carbon dioxide, $C{{O}_{2}}$, and nitrogen gas${{N}_{2}}$, which are very stable and releases high amount of energy.
As the name suggests TNT, trinitrotoluene, is made from toluene. This toluene is treated with nitric and sulphuric acid mixtures to obtain TNT. The structure of TNT is as follows:

Clearly, the structure of TNT does not have thorium, phthalic acid or tin, therefore, it is made of toluene.
Hence, the explosive TNT is made from toluene.
Note:
TNT is used as an explosive for bombs, grenades and in military uses, also for collapsing buildings. The gases that are evolved are considered as very poisonous and can lead to serious injuries and illnesses in humans. The explosion creates a high amount of shock waves that result in mass deterioration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




