
The equivalent mass of an element is 4. Its chloride has vapour density 59.25. Then the valency of the element is:
(A) 4
(B) 2
(C) 3
(D) 1
Answer
588.6k+ views
Hint: First of all, find out the molecular weight of the element. And then as per the given question, the equivalent mass is already given from which you can get the valency of the element.
Complete step by step solution:
Let us consider the compound to be $\text{MC}{{\text{l}}_{X}}$, where ‘M’ is the element and X is the valency of the element M (Refer to the image).
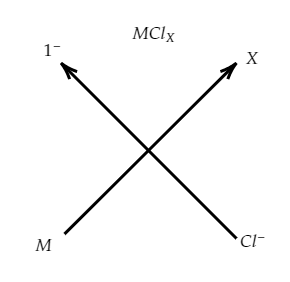
Thus, the formula of the compound is $\text{MC}{{\text{l}}_{X}}$.
Equivalent mass of an element is 4.
Vapour density of its chloride is 59.25.
And we have to find the valency of the element.
Using formula,
Molecular\ weight = $\text{2 }\times \text{ Vapour density}$, from which we can find out the molecular weight of the compound.
So,
Molecular weight of $\text{MC}{{\text{l}}_{X}}$ = $\text{2 }\times \text{ 59}\text{.5}$= 118.5 …(i)
As we know, the molecular weight can also be calculated by summing the atomic weight of the atoms making up the substance’s molecular formula.
We know, the atomic mass of Cl is 35.5.
So here the molecular weight of $\text{MC}{{\text{l}}_{X}}$ can be calculated as,
Molecular weight of $\text{MC}{{\text{l}}_{X}}$ = $\text{M }\times \text{ 1 + X }\times \text{ 35}\text{.5}$ …(ii)
where, M = Atomic mass of element M. X = Number of atoms of Cl.
Here, we can put 118.5 from equation (i) in equation (ii).
So, $\text{M + 35}\text{.5X = 118}\text{.5}$. …(iii)
Using formula,
$Equivalentweight=\dfrac{Atomic\text{ }mass}{Valency}$
So,
$4=\dfrac{M}{X}$ [Atomic weight = M from equation (ii) and equivalent weight is already given]
Therefore, $\text{M = 4X}$ …(iv)
Now, putting the value of M in equation (iii), we can find out the valency of the element.
Then,
$\text{M + 35}\text{.5X = 118}\text{.5}$
Here, putting the value of M from equation (iv) the equation will be,
$\text{4X+35}\text{.5X=118}\text{.5}$
So, $\text{39}\text{.5X=118}\text{.5}$
And the value of X will be,
$\text{X=}\dfrac{118.5}{39.5}$
Therefore, $\text{X= 3}$
So, the valency of the element will be 3.
Hence, the correct option is (C).
Note: The possibility for the mistake is that you can choose the option (D) considering the formula as $\text{MC}{{\text{l}}_{X}}$. Considering the crisscross method, the valency of the element M would be X which we have to find out.
Complete step by step solution:
Let us consider the compound to be $\text{MC}{{\text{l}}_{X}}$, where ‘M’ is the element and X is the valency of the element M (Refer to the image).

Thus, the formula of the compound is $\text{MC}{{\text{l}}_{X}}$.
Equivalent mass of an element is 4.
Vapour density of its chloride is 59.25.
And we have to find the valency of the element.
Using formula,
Molecular\ weight = $\text{2 }\times \text{ Vapour density}$, from which we can find out the molecular weight of the compound.
So,
Molecular weight of $\text{MC}{{\text{l}}_{X}}$ = $\text{2 }\times \text{ 59}\text{.5}$= 118.5 …(i)
As we know, the molecular weight can also be calculated by summing the atomic weight of the atoms making up the substance’s molecular formula.
We know, the atomic mass of Cl is 35.5.
So here the molecular weight of $\text{MC}{{\text{l}}_{X}}$ can be calculated as,
Molecular weight of $\text{MC}{{\text{l}}_{X}}$ = $\text{M }\times \text{ 1 + X }\times \text{ 35}\text{.5}$ …(ii)
where, M = Atomic mass of element M. X = Number of atoms of Cl.
Here, we can put 118.5 from equation (i) in equation (ii).
So, $\text{M + 35}\text{.5X = 118}\text{.5}$. …(iii)
Using formula,
$Equivalentweight=\dfrac{Atomic\text{ }mass}{Valency}$
So,
$4=\dfrac{M}{X}$ [Atomic weight = M from equation (ii) and equivalent weight is already given]
Therefore, $\text{M = 4X}$ …(iv)
Now, putting the value of M in equation (iii), we can find out the valency of the element.
Then,
$\text{M + 35}\text{.5X = 118}\text{.5}$
Here, putting the value of M from equation (iv) the equation will be,
$\text{4X+35}\text{.5X=118}\text{.5}$
So, $\text{39}\text{.5X=118}\text{.5}$
And the value of X will be,
$\text{X=}\dfrac{118.5}{39.5}$
Therefore, $\text{X= 3}$
So, the valency of the element will be 3.
Hence, the correct option is (C).
Note: The possibility for the mistake is that you can choose the option (D) considering the formula as $\text{MC}{{\text{l}}_{X}}$. Considering the crisscross method, the valency of the element M would be X which we have to find out.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




