
The enone $ {\text{(X = }}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}{\text{O)}} $ which shows geometrical isomerism are:
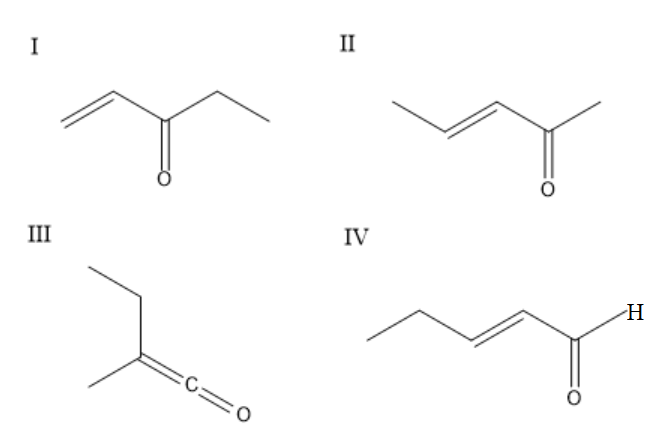
(A) $ \;{{I\;and IV}} $
(B) $ {{II\;and IV}} $
(C) $ {{III\;and IV}} $
(D) $ {{II only}} $

Answer
516.6k+ views
Hint: The chemical species which have the same type and same quantity of atoms as another species but a different geometric structure are called geometric isomers. These atoms or the groups exhibit different spatial arrangements on both sides of chemical bond or ring structure. The most common type of geometric isomers arise from a ring structure or from a double bond. These types of isomers are called cis and trans isomers.
Complete answer:
To identify the geometrical isomers, check whether there are two different groups on the left-hand side of the bond and two different groups on the right-hand side of the bond. Consider the first compound, it will not show geometrical isomerism because the carbon-carbon double bond is at the end and it will have similar groups on one side. The second compound exhibits geometrical isomerism and can exist in cis and trans forms. The third compound will not exhibit geometrical isomerism because carbon is attached to only one group on one side. The fourth group exhibits geometrical isomerism and exists in cis and trans forms. The second compound and fourth compound show geometric isomerism.
Therefore, option B is the correct answer.
Note:
Geometrical isomerism arises because of the restricted rotation around the carbon-carbon double bond. Because of this hindered rotation around the carbon-carbon double bond, the relative positions of groups or atoms that are attached to the carbon-carbon double bond are fixed. An enone consists of an alkene which is conjugated to a ketone.
Complete answer:
To identify the geometrical isomers, check whether there are two different groups on the left-hand side of the bond and two different groups on the right-hand side of the bond. Consider the first compound, it will not show geometrical isomerism because the carbon-carbon double bond is at the end and it will have similar groups on one side. The second compound exhibits geometrical isomerism and can exist in cis and trans forms. The third compound will not exhibit geometrical isomerism because carbon is attached to only one group on one side. The fourth group exhibits geometrical isomerism and exists in cis and trans forms. The second compound and fourth compound show geometric isomerism.
Therefore, option B is the correct answer.
Note:
Geometrical isomerism arises because of the restricted rotation around the carbon-carbon double bond. Because of this hindered rotation around the carbon-carbon double bond, the relative positions of groups or atoms that are attached to the carbon-carbon double bond are fixed. An enone consists of an alkene which is conjugated to a ketone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




