
The end product (Z) of the given reaction is:
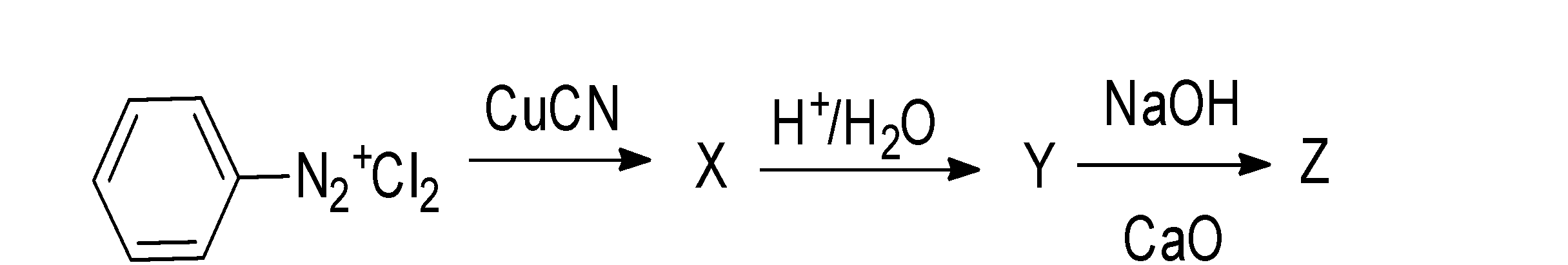
(a) a cyanide
(b) a carboxylic acid
(c) an amine
(d) arene
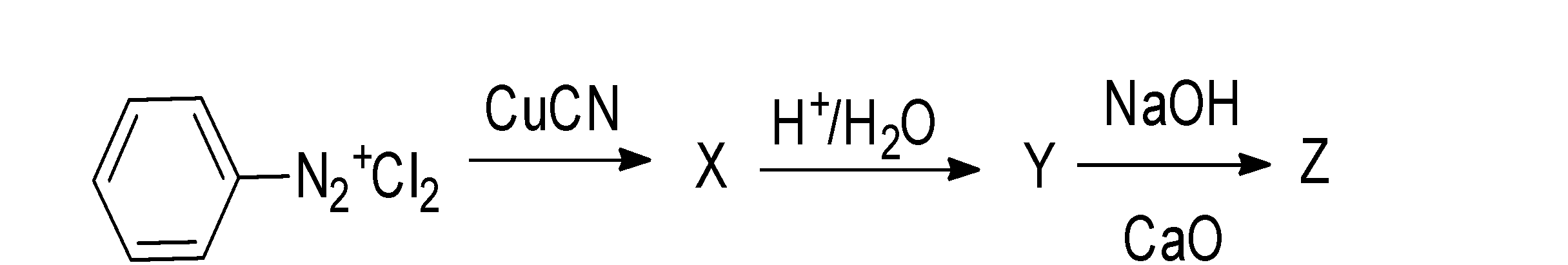
Answer
504.3k+ views
Hint: When benzene diazonium chloride is treated with copper cyanide i.e. CuCN , it forms a nitrile which on hydrolysis, gives an acid and when this acid is treated with the soda lime i.e. (sodium hydroxide and calcium oxide), it undergoes decarboxylation reaction and results in the formation of an aromatic compound. Now you can easily answer the given statement accordingly. Solve it.
Complete answer:
When the $N_{2}^{+}C{{l}^{-}}$ group is attached to the benzene ring, then the compound is said to be benzene diazonium chloride. When this benzene diazonium chloride is made it undergoes a reaction with the copper cyanide, which results in the formation of nitrile compounds i.e. benzonitrile(X). The reaction takes place as;
$Ph-N=N-Cl\xrightarrow{CuCN}Ph-CN$
When this benzonitrile is made to undergo hydrolysis , it results in the formation of an acid i.e. benzoic acid(Y). The reaction occurs as;
$Ph-CN\xrightarrow{{{H}^{+}}/{{H}_{2}}O}Ph-COOH$
And when this benzoic acid is made to react with the sodium hydroxide and calcium oxide i.e. soda lime, it undergoes decarboxylation reaction and results in the formation of an aromatic compound, arene i.e. benzene(Z). the reaction occurs as;
$Ph-COOH\xrightarrow{{{H}^{+}}/{{H}_{2}}O}Ph-H$
Thus, the overall reaction occur as;
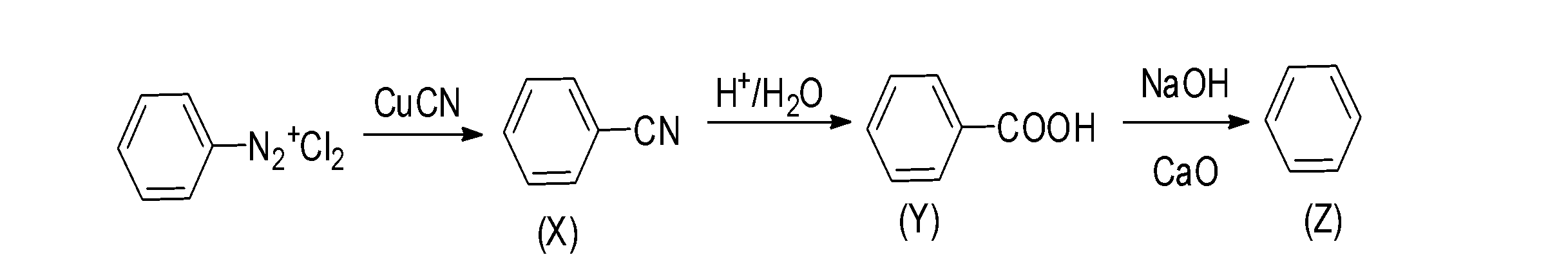
And the end product (Z) is arene.
So, the correct answer is “Option d”.
Note:
Decarboxylation reactions are those reactions in which there is loss of carbon dioxide molecule when a compound( aromatic compound) undergoes reaction with the soda lime (i.e. sodium hydroxide and calcium oxide).
Complete answer:
When the $N_{2}^{+}C{{l}^{-}}$ group is attached to the benzene ring, then the compound is said to be benzene diazonium chloride. When this benzene diazonium chloride is made it undergoes a reaction with the copper cyanide, which results in the formation of nitrile compounds i.e. benzonitrile(X). The reaction takes place as;
$Ph-N=N-Cl\xrightarrow{CuCN}Ph-CN$
When this benzonitrile is made to undergo hydrolysis , it results in the formation of an acid i.e. benzoic acid(Y). The reaction occurs as;
$Ph-CN\xrightarrow{{{H}^{+}}/{{H}_{2}}O}Ph-COOH$
And when this benzoic acid is made to react with the sodium hydroxide and calcium oxide i.e. soda lime, it undergoes decarboxylation reaction and results in the formation of an aromatic compound, arene i.e. benzene(Z). the reaction occurs as;
$Ph-COOH\xrightarrow{{{H}^{+}}/{{H}_{2}}O}Ph-H$
Thus, the overall reaction occur as;

And the end product (Z) is arene.
So, the correct answer is “Option d”.
Note:
Decarboxylation reactions are those reactions in which there is loss of carbon dioxide molecule when a compound( aromatic compound) undergoes reaction with the soda lime (i.e. sodium hydroxide and calcium oxide).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




