
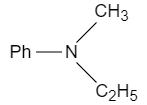
The end product $(2)$ of the reaction sequence:
A.
B.
C.
D.





Answer
546.3k+ views
Hint: In this question, Heinsberg test is used to distinguish between primary, secondary and tertiary amines. In this test, amine is reacted with benzene carbonyl chloride.
$LiAl{{H}_{4}}$ acts as a strong reducing agent .
Complete step by step answer:
Lithium aluminium hydride is an inorganic compound having formula, $LiAl{{H}_{4}}$ . It acts as a strong reducing agent which is used for the reduction of amides, esters, carboxylic acids to alcohols and ketones. It also reduces alkyl halides to alkane.
Benzene carbonyl chloride having the formula ${{C}_{6}}{{H}_{5}}CPOCl$ is a colorless fuming liquid. It is an organochlorine compound which is mainly used for the production of peroxides. It is used for the preparation of dyes, perfumes and raisins.
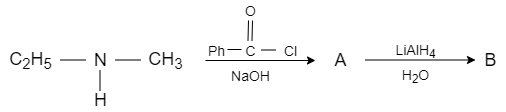
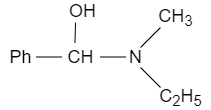
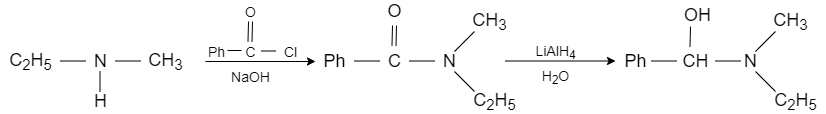
When ethyl methyl amine is reacted with benzene carbonyl chloride in presence of sodium hydroxide, the benzoyl radical from benzene carbonyl chloride attacks on acidic hydrogen and forms benzoyl ethyl methyl amide, which reacts with lithium aluminium hydride that acts as a reducing agent and converts it into $N-$ ethyl $-N-$ methyl $-1-$ hydroxy $-1-$ phenylmethanamine. It converts the carbonyl carbon into alcohol.
So, the correct answer is Option C.
Additional information:
We can prepare benzoyl chloride from benzo trichloride with water or benzoic acid.
Lithium aluminum hydride is a colorless solid, but due to contamination, their commercial samples are grey. Lithium aluminium hydride can be purified by recrystallisation from diethyl ether. It reacts violently with water.
$LiAl{{H}_{4}}+4{{H}_{2}}O\to LiOH+Al{{(OH)}_{3}}+4{{H}_{2}}$
This reaction can be used for the production of hydrogen in the laboratory.
Note: In conclusion, the ethyl methyl amine reacted with benzene carbonyl chloride with sodium hydroxide and a strong reducing agent with water, it results in the formation of $N-$ ethyl $-N-$ methyl $-1-$ hydroxy $-1-$ phenylmethanamine.
$LiAl{{H}_{4}}$ acts as a strong reducing agent .
Complete step by step answer:
Lithium aluminium hydride is an inorganic compound having formula, $LiAl{{H}_{4}}$ . It acts as a strong reducing agent which is used for the reduction of amides, esters, carboxylic acids to alcohols and ketones. It also reduces alkyl halides to alkane.
Benzene carbonyl chloride having the formula ${{C}_{6}}{{H}_{5}}CPOCl$ is a colorless fuming liquid. It is an organochlorine compound which is mainly used for the production of peroxides. It is used for the preparation of dyes, perfumes and raisins.
When ethyl methyl amine is reacted with benzene carbonyl chloride in presence of sodium hydroxide, the benzoyl radical from benzene carbonyl chloride attacks on acidic hydrogen and forms benzoyl ethyl methyl amide, which reacts with lithium aluminium hydride that acts as a reducing agent and converts it into $N-$ ethyl $-N-$ methyl $-1-$ hydroxy $-1-$ phenylmethanamine. It converts the carbonyl carbon into alcohol.

So, the correct answer is Option C.
Additional information:
We can prepare benzoyl chloride from benzo trichloride with water or benzoic acid.
Lithium aluminum hydride is a colorless solid, but due to contamination, their commercial samples are grey. Lithium aluminium hydride can be purified by recrystallisation from diethyl ether. It reacts violently with water.
$LiAl{{H}_{4}}+4{{H}_{2}}O\to LiOH+Al{{(OH)}_{3}}+4{{H}_{2}}$
This reaction can be used for the production of hydrogen in the laboratory.
Note: In conclusion, the ethyl methyl amine reacted with benzene carbonyl chloride with sodium hydroxide and a strong reducing agent with water, it results in the formation of $N-$ ethyl $-N-$ methyl $-1-$ hydroxy $-1-$ phenylmethanamine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




