
The electrovalent linkage is present in
(A) ${{O}_{2}}$
(B) $CC{{l}_{4}}$
(C) $CHC{{l}_{3}}$
(D) NaBr
Answer
533.7k+ views
Hint: When oppositely charged ions of a chemical compound are bonded by the electrostatic attraction between them, the linkage formed is known as electrovalent linkage. These linkages are also known as ionic bonds.
Complete answer: To find out which of the given compounds have an electrovalent linkage or ionic bonding, we first need to know how the atoms in the following compounds are bonded to each other.
${{O}_{2}}$: In an oxygen molecule, the oxygen atoms have 6 valence electrons.
So, to complete the octet of each atom, the oxygen atom shares two electrons with each other to form the double bond as follows
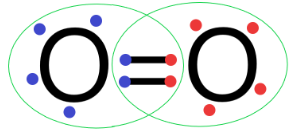
So, we can see that atoms in oxygen molecules are linked by covalent bonds.
$CC{{l}_{4}}$: In a carbon tetrachloride molecule, the carbon atom has 4 valence electrons and the chlorine atom has 7 valence electrons.
So, to complete the octet of each atom, the carbon atom shares 1 electron with each chlorine atom to form the bonds as follows:
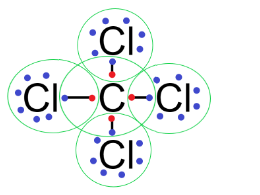
So, we can see that atoms in $CC{{l}_{4}}$molecules are linked by covalent bonds.
$CHC{{l}_{3}}$: In a carbon tetrachloride molecule, the carbon atom has 4 valence electrons, the hydrogen atom has 1 valence electron and the chlorine atom has 7 valence electrons.
So, to complete the octet of each atom, the carbon atom shares 1 electron with each chlorine atom and hydrogen atom to form the bonds as follows:
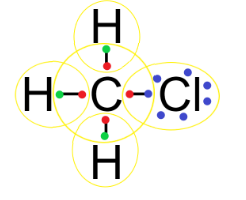
So, we can see that atoms in $CHC{{l}_{3}}$ molecules are linked by covalent bonds.
NaBr: In the sodium bromide molecule, the sodium atom has 1 valence electron, and the bromine atom has 7 valence electrons.
So, to complete the octet of each atom, the sodium atom loses 1 electron to the bromine atom to form the bonds as follows:
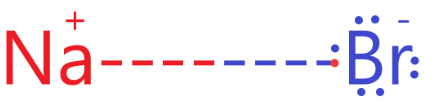
So, we can see that atoms in NaBr molecules are linked by ionic bonds or electronic linkage.
So, the correct answer is an option (D) NaBr.
Note: It should be noted that the main difference between an ionic bond and a covalent bond is that in covalent bonding the electrons are shared between the atoms to fulfill the octet configuration of each atom, whereas in ionic bonds the electrons are lost by an atom and gained by another atom to achieve the most stable configuration.
Complete answer: To find out which of the given compounds have an electrovalent linkage or ionic bonding, we first need to know how the atoms in the following compounds are bonded to each other.
${{O}_{2}}$: In an oxygen molecule, the oxygen atoms have 6 valence electrons.
So, to complete the octet of each atom, the oxygen atom shares two electrons with each other to form the double bond as follows

So, we can see that atoms in oxygen molecules are linked by covalent bonds.
$CC{{l}_{4}}$: In a carbon tetrachloride molecule, the carbon atom has 4 valence electrons and the chlorine atom has 7 valence electrons.
So, to complete the octet of each atom, the carbon atom shares 1 electron with each chlorine atom to form the bonds as follows:

So, we can see that atoms in $CC{{l}_{4}}$molecules are linked by covalent bonds.
$CHC{{l}_{3}}$: In a carbon tetrachloride molecule, the carbon atom has 4 valence electrons, the hydrogen atom has 1 valence electron and the chlorine atom has 7 valence electrons.
So, to complete the octet of each atom, the carbon atom shares 1 electron with each chlorine atom and hydrogen atom to form the bonds as follows:

So, we can see that atoms in $CHC{{l}_{3}}$ molecules are linked by covalent bonds.
NaBr: In the sodium bromide molecule, the sodium atom has 1 valence electron, and the bromine atom has 7 valence electrons.
So, to complete the octet of each atom, the sodium atom loses 1 electron to the bromine atom to form the bonds as follows:

So, we can see that atoms in NaBr molecules are linked by ionic bonds or electronic linkage.
So, the correct answer is an option (D) NaBr.
Note: It should be noted that the main difference between an ionic bond and a covalent bond is that in covalent bonding the electrons are shared between the atoms to fulfill the octet configuration of each atom, whereas in ionic bonds the electrons are lost by an atom and gained by another atom to achieve the most stable configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




