
The electrostatic force of attraction between cation and anion is called___________.
Answer
520.8k+ views
Hint :cation and anions are ions both having different net electrical charges. Cations are ions having a net positive charge and anions are ions having a net negative charge. As both ions have opposite charges, they attract each other and form bonds.
Complete Step By Step Answer:
When cation and anion come close to each other, having opposite net electric charges they both experience a force of attraction to form a bond, this force of attraction is called an electrostatic force of attraction. It is just like how the two opposite poles of a magnet attract each other. And the bond that the cation and anion form is an ionic bond. This force of attraction holds them together as a unit.
The ionic bond which is formed between the cation and anion is very strong. In this, there is a complete transfer of electrons from one molecule to the other. There is a huge electronegativity difference between the two ions when there is a formation of this bond.
To understand this we can take an example of a simple molecule: $ NaCl $
So in $ Na $ there is $ 1 $ electron in the outermost shell and in $ Cl $ there are $ 7 $ electrons in the outermost shell. It will be difficult for $ Cl $ to transfer $ 7 $ electrons so $ Na $ transfers it $ 1 $ electron to $ Cl $ and there is a bond formed between them.
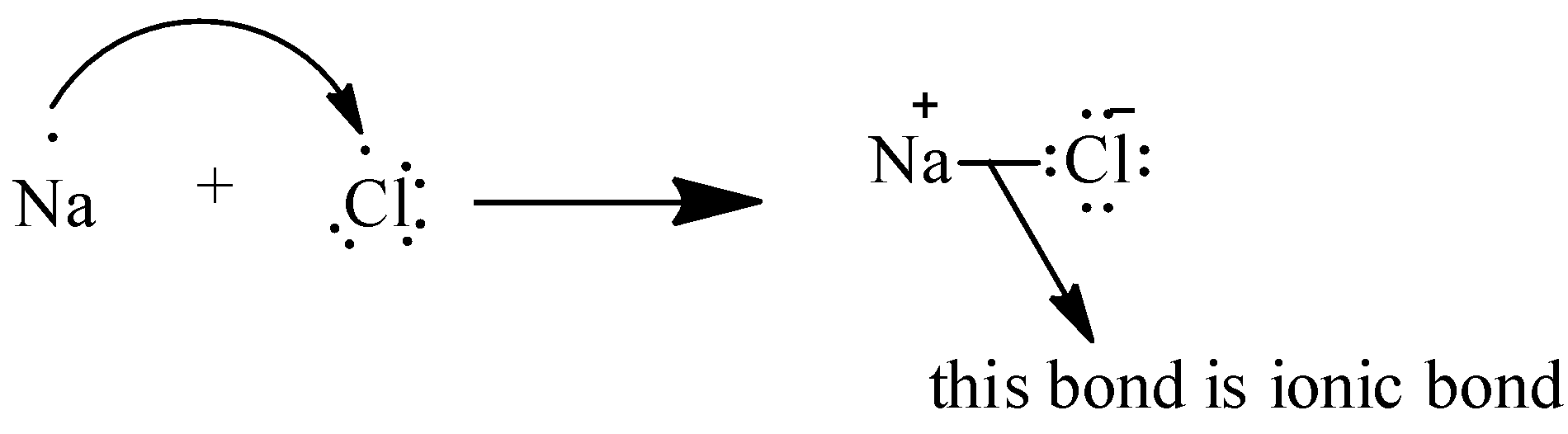
As we can see in the above picture, bond formation between $ Na $ and $ Cl $ which results in the formation of $ NaCl $ molecule. And the force of attraction with which the bond is formed is called the electrostatic force of attraction.
Note :
In physics, this electrostatic force of attraction is also called coulomb’s law, named after French physicist Charles-Augustin de Coulomb. And this force of attraction between two oppositely charged bodies is calculated using formula $ F = k\difrac{{{q_1}{q_2}}}{{{r^2}}} $ .
Complete Step By Step Answer:
When cation and anion come close to each other, having opposite net electric charges they both experience a force of attraction to form a bond, this force of attraction is called an electrostatic force of attraction. It is just like how the two opposite poles of a magnet attract each other. And the bond that the cation and anion form is an ionic bond. This force of attraction holds them together as a unit.
The ionic bond which is formed between the cation and anion is very strong. In this, there is a complete transfer of electrons from one molecule to the other. There is a huge electronegativity difference between the two ions when there is a formation of this bond.
To understand this we can take an example of a simple molecule: $ NaCl $
So in $ Na $ there is $ 1 $ electron in the outermost shell and in $ Cl $ there are $ 7 $ electrons in the outermost shell. It will be difficult for $ Cl $ to transfer $ 7 $ electrons so $ Na $ transfers it $ 1 $ electron to $ Cl $ and there is a bond formed between them.
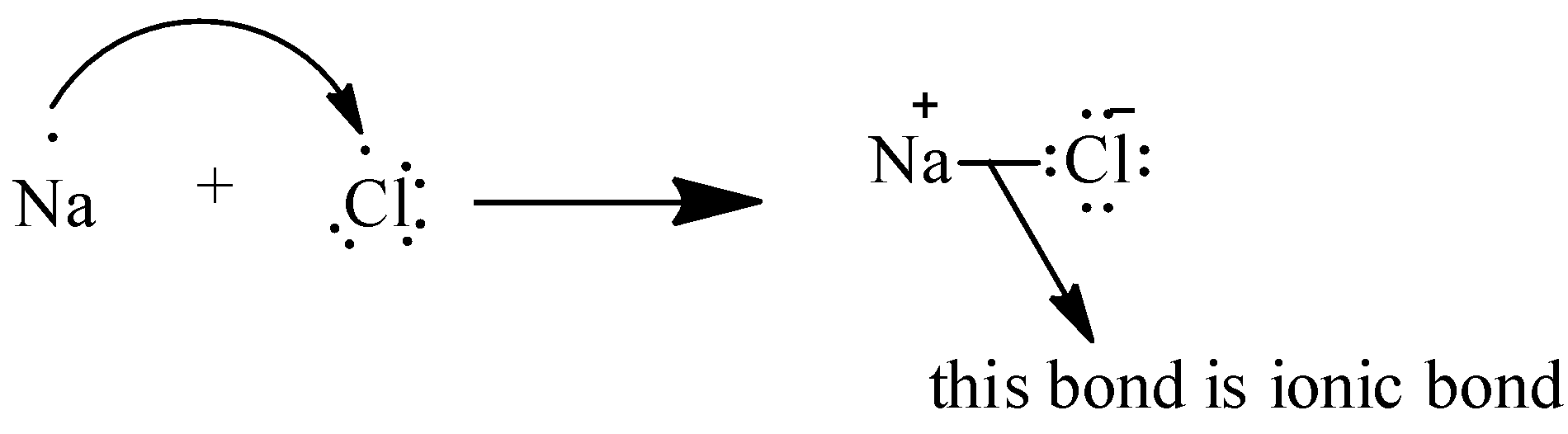
As we can see in the above picture, bond formation between $ Na $ and $ Cl $ which results in the formation of $ NaCl $ molecule. And the force of attraction with which the bond is formed is called the electrostatic force of attraction.
Note :
In physics, this electrostatic force of attraction is also called coulomb’s law, named after French physicist Charles-Augustin de Coulomb. And this force of attraction between two oppositely charged bodies is calculated using formula $ F = k\difrac{{{q_1}{q_2}}}{{{r^2}}} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




