
The electrons are more likely to be found:
A. in the region a and b
B. only in region c
C. in the region a and c
D. only in the region a

Answer
577.8k+ views
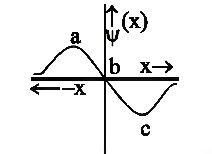
Hint:. The graph given in the question shows us the relation between $\psi $ and x. But, the probability depends on $P=4\pi {{x}^{2}}{{[\psi (x)]}^{2}}$. So, the graph needs to be converted to the respective square form and then we can find the required answer.
Complete step by step answer:
An atomic orbital is defined as the place inside an atom where the electron is likely to be present with a probability of 90% of the time. The probability of finding the electrons in a specified orbital depends on the wave function $\psi $. To be precise, the square of the wave function i.e ${{\psi }^{2}}$ can be used to find the electron density probability in a given orbital of an atom.
According to our graph, we have not been given the squares. However, we can still find the required probability by transforming the graph. We know that whenever a number is squared, be it a positive or negative integer, it is always positive (after squaring). Similarly, if the x-axis and y-axis of the graph, both are squared, then the negative parts flip themselves and become positive. That means, in perspective of y-axis, the region c gets inverted to the top and in perspective of x-axis, the region a gets flipped to the right.
In the final graph, we obtain 2 peaks, represented by the region a and c, where the probability of finding an electron is the maximum.
So, the correct answer is “Option C”.
Note: Generally, the 1s orbital has the maximum probability of finding an electron. In order to solve our question, we can simply mirror both the x-axis and y-axis and draw out our conclusion from the resultant graph. In case we had a negative peak in our final graph, that region would be the area where the probability of finding an electron is the least.
Complete step by step answer:
An atomic orbital is defined as the place inside an atom where the electron is likely to be present with a probability of 90% of the time. The probability of finding the electrons in a specified orbital depends on the wave function $\psi $. To be precise, the square of the wave function i.e ${{\psi }^{2}}$ can be used to find the electron density probability in a given orbital of an atom.
According to our graph, we have not been given the squares. However, we can still find the required probability by transforming the graph. We know that whenever a number is squared, be it a positive or negative integer, it is always positive (after squaring). Similarly, if the x-axis and y-axis of the graph, both are squared, then the negative parts flip themselves and become positive. That means, in perspective of y-axis, the region c gets inverted to the top and in perspective of x-axis, the region a gets flipped to the right.
In the final graph, we obtain 2 peaks, represented by the region a and c, where the probability of finding an electron is the maximum.
So, the correct answer is “Option C”.
Note: Generally, the 1s orbital has the maximum probability of finding an electron. In order to solve our question, we can simply mirror both the x-axis and y-axis and draw out our conclusion from the resultant graph. In case we had a negative peak in our final graph, that region would be the area where the probability of finding an electron is the least.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




